
R Douglas Fields
https://www.researchgate.net/publication/266624474_Myelination_of_the_Nervous_System_Mechanisms_and_Functions
Myelination of the Nervous System: Mechanisms and Functions
DOI: 10.1146/annurev-cellbio-100913-013101 · Source: PubMed
Myelination of the Nervous System: Mechanisms and Functions (PDF Download Available). Available from:
https://www.researchgate.net/publication/266624474_Myelination_of_the_Nervous_System_Mechanisms_and_Functions [accessed Feb 14 2018].
8
9
11
12
13
14
15
19
20
21
22
PLP
23
(Vallitsevan teorian kehittämisen pioneerit ja suuret nimet merkitty punaisella ja linkitetty, kriteerinä erityisesti, että R. D. Fields on kertonut tutkimuksista aiheen perusteoksissaan, HM, suomalaiset henkilöt ja tutkimukset merkitty sinisellä.)
26
28
31
She’s 17 years old and already helping patients. Meet the winner of one of the country’s most prestigious science fairs.
Indrani Das has been fascinated with brain injuries since her freshman year of high school, when she learned that their effects can be devastating and irreversible.
Later, her fascination evolved into a full-fledged research project. Das, now 17 and a senior at the Academy for Medical Science Technology in Hackensack, N.J., ex-plored how brain damage occurs, examining a process called astrogliosis, which can lead to the excess production of a toxin that can damage neurons. If she and other researchers could better understand how brain damage occurs, perhaps they could figure out how to slow or reverse the process.
“My work centers on repairing the behavior of supporting cells to prevent neuron in-jury and death,” Das said. “It was really that shock of what it can do to a person that pushed me to work” on research involving brain injuries.
Das’s project, which explores the role of brain cells called astrocytes in the death of neurons, was awarded the top prize and $250,000 at the Regeneron Science Talent Search.
Das bested thousands of high school scientists from across the country. The talent search selected 40 finalists, who traveled last week to D.C., where a selection com-mittee grilled them on their work and put them through the wringer, testing their grasp of scientific concepts and their ability to solve problems.
Four of the finalists were from the Washington area: Prathik Naidu of Thomas Jef-ferson High School for Science and Technology in Fairfax County; David Rekhtman of Walt Whitman High School in Bethesda; and Sambuddha Chattopadhyay and Rohan Dalvi, both of Montgomery Blair High School in Silver Spring.
Naidu placed seventh, taking home $70,000 for his project, which created 3-D models of the genetics of cancer cells, using a computer program he built.
The Science Talent Search, previously sponsored by Intel, is one of the best-known and among the most competitive science fairs for young researchers. This year, the talent search gave out $1.8 million to 40 finalists, much of which will go to cover college tuition for the budding researchers.
[These teens are working to cure cancer and solve the mysteries of the universe]
Maya Ajmera, president and chief executive of the Society for Science and the Pub-lic, said the finalists are often well-rounded and driven by their desire to make the world a better place, an altruism that is reflected in extracurricular activities.
Das, who hails from Orendell, N.J., recently became a certified emergency medical technician and is already working with patients, helping to transport them to hospitals. While she is deeply fascinated by research, she also hopes to become a practicing physician so she can work with patients.
“I would say my happiest time is when I’m with my patients,” Das said. “I love connecting with people and understanding how I can help them. It keeps me human.”
She plans to use the prize money to help pay for college and medical school.

Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4439926/
Physiological Function of Microglia
Role of Myelin Plasticity in Oscillations and Synchrony of Neuronal Activity
https://science.howstuffworks.com/life/inside-the-mind/human-brain/remember-birth.htm
Can a person remember being born?
Think back to your earliest memory. Perhaps images of a birthday party or scenes from a family vacation come to mind. Now think about your age when that event oc-curred. Chances are that earliest recollection extends no further back than your third birthday. In fact, you can probably come up with only a handful of memories from be-tween the ages of 3 and 7, although family photo albums or other cues may trigger more.
Psychologists refer to this inability of most adults to remember events from early life, including their birth,as childhood amnesia.Sigmund Freud first coined the term infan- tile amnesia, now more broadly referred to as childhood amnesia,as early as 1899 to explain his adult patients' scarcity of childhood memories [source: Rapaport]. Freud proposed that people use it as a means of repressing traumatic, and often sexual, urgings during that time. To block those unconscious drives of the id, Freud claimed that humans create screen memories, or revised versions of events, to protect the conscious ego. "
Puutaheinää. Oikea selitys täällä:
CG: " More than a century later, researchers have yet to pin down a precise expla-nation for why childhood amnesia occurs. Only in the last 20 years have people in-vestigated children's,rather than adults',memory capabilities in search of the answer. This research has brought with it a new batch of questions about the nuances of young children's memory.
For a long time, the rationale behind childhood amnesia rested on the assumption that the memory-making parts of babies' brains were undeveloped. Then, around age 3, children's memory capabilities rapidly accelerate to adult levels. "
T.: Näinkin voi sanoa. Mutta kielen oppiminen nimenomaan luo myös ne aivojen ka-pasiteetit, joille ihmisen ajattelu perustuu. Tämän huomasi jo 1700-luvulla englanti-lainen psykiatri David Hartley alun perin fyysiä aivoja tutkimalla vastikään kehitetyllä mikroskoopilla.
CC: " However, psychologists have discovered that children as young as 3 months old and 6 months old can form long-term memories. "
T.: Yes. Suoria ehdollisia refleksejä. Niitä ei muisteta sellaisinaan, vaan muiden, kie-lellisten muistojen yhteydessä,joiden muodostumiseen ne vaikuttavat ollen alun perin näiden rakennuspalikkoina, jotka kootaan kielellisesti muistettaviksi muistoiksi.
https://science.howstuffworks.com/life/inside-the-mind/human-brain/remember-birth1.htm
... CC: " The difference comes in which memories stick around. For instance, it appears that babies are born with more intact implicit, or unconscious, memories. "
T.: Ehdottomia refleksejä EI pidä nimittää lainkaan "MUISTOIKSI" eikä tajunnaksi, koska ne eivät ole sitä. Suorat ehdolliset refleksit sellaisenaan eivät myöskään ole tajuntaa. Ne ovat sitä, millä esimerkiksi simpanssi käyttäytyy, ja mekin kävelemme ja juoksemme pystyssä ja pidämme myös polkupyörää ja moottoripyörää pystyssä ajaessamme. (Koska muuten tekoäly oppii ajamaan moottoripyörällä? Sitä ennen ei kannata puhua TIETOISESTA tekoälystä, sillä tuo tehtävä on HELPOMPI.)
CC: " At the same time the explicit, or episodic, memory that records specific events does not carry information over that three-year gap, explaining why people do not remember their births.
But why does this happen, and what changes take place in those first years? And if we can form memories as babies, why don't we retain them into adulthood?
On the next page, we'll take a closer look at a baby's brain to find out the answer.
Memory Encoding in Children
To form memories,humans must create synapses,or connections between brain cells (linkitys lähteen, T.), that encode sensory information from an event into our memory. From there, our brains organize that information into categories and link it to other similar data, which is called consolidation. In order for that memory to last, we must periodically retrieve these memories and retrace those initial synapses, reinforcing those connections.
Studies have largely refuted the long-held thinking that babies cannot encode infor-mation that forms the foundation of memories. For instance, in one experiment invol-ving 2- and 3-month-old infants, the babies' legs were attached by a ribbon to a mo-bile [source: Hayne]. By kicking their legs, the babies learned that the motion caused the mobile to move. Later, placed under the same mobile without the ribbon, the in-fants remembered to kick their legs. When the same experiment was performed with 6-month-olds, they picked up the kicking relationship much more quickly, indicating that their encoding ability must accelerate gradually with time, instead of in one significant burst around 3 years old.
This memory encoding could relate to a baby's development of the prefrontal cortex at the forehead. This area, which is active during the encoding and retrieval of expli-cit memories, is not fully functional at birth [source: Newcombe et al]. However, by 24 months, the number of synapses in the prefrontal cortex has reached adult levels [source: Bauer].
Also, the size of the hippocampus at the base of the brain steadily grows until your second or third year [source: Bauer]. This is important because the hippocampus determines what sensory information to transfer into long-term storage.
https://science.howstuffworks.com/life/inside-the-mind/human-brain/remember-birth2.htm
"... But what about implicit memory? Housed in the cerebellum, implicit memory is essential for newborns, allowing them to associate feelings of warmth and safety with the sound of their mother's voice and instinctively knowing how to feed. Confirming this early presence,studies have revealed few developmental changes in implicit me- mory as we age [source: Newcombe et al]. Even in many adult amnesia cases, impli-cit skills such as riding a bicycle or playing a piano often survive the brain trauma.
Now we know that babies have a strong implicit memory and can encode explicit ones as well, which indicates that childhood amnesia may stem from faulty explicit memory retrieval. Unless we're thinking specifically about a past event,it takes some sort of cue to prompt an explicit memory in all age groups [source: Bauer]. Up next, find out what those cues are. "
T.: Kyse ei ole vain "palautusvirheestä",vaan siitä, että ihmiselle ominainen kielellis-rakenteinen tajunta on vasta muodostumassa ympäristöstä riippumattomalla tavalla ajatuksellisesti mieleenpalautettavissa olevine muistoineen.
CC: " Language and Sense of Self in Memory-Making
Our earliest memories may remain blocked from our consciousness because we had no language skills at that time.A 2004 study traced the verbal development in 27- and 39-month old boys and girls as a measure of how well they could recall a past event. The researchers found that if the children didn't know the words to describe the event when it happened, they couldn't describe it later after learning the appropriate words [source: Simcock and Hayne].
Verbalizing our personal memories of events contributes to our autobiographical memories. These types of memories help to define our sense of self and relationship to people around us. Closely linked to this is the ability to recognize yourself. Some researchers have proposed that children do not develop self-recognition skills and a personal identity until 16 or 24 months [source: Fivush and Nelson].
In addition, we develop knowledge of our personal past when we begin to organize memories into a context.
Many preschool-age children can explain the different parts of an event in sequential order, such as what happened when they went to a circus. But it isn't until their fifth year that they can understand the ideas of time and the past and are able to place that trip to the circus on a mental time line [source: Fivush and Nelson].
Parents play a pivotal role in developing children's autobiographical memory as well. Research has shown that the way parents verbally recall memories with their small children correlates to those children's narrative style for retelling memories later in life. In other words, children whose parents tell them about past events, such as birth- day parties or trips to the zoo, in detail will be more likely to vividly describe their own memories [source: Urshwa]. Interestingly, autobiographical memory also has a cultu-ral component, with Westerners' personal memories focusing more on themselves and Easterners remembering themselves more in group contexts [source: Urshwa].
More detailed explanations exist regarding childhood amnesia. But brain structure, language and sense of self are its foundation. To learn more about amnesia and memory, don't forget to read the links below. ...
Primal Healing
Flying in the face of childhood amnesia research,some people claim to recall prever- bal memories and even recollections from the womb. One form of psychoanalysis, called primal healing, focuses on traumatic early memories similar to Sigmund Freud's theory of repressed and screen memories.Primal therapy links people's pre- sent pain with the pain of birth,taking patients back to the memory of their own birth in a process referred to as rebirthing. However,in spite of anecdotal evidence, no scien- tific study has verified the authenticity of these rebirthing experiences [source:Eisner]. "
Related HowStuffWorks Articles
Dr. Douglas Fields: "Exploring New Frontiers in Neuroscience" | Talks at Google
Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons
Abstract
The myelin sheath on vertebrate axons is critical for neural impulse transmission, but whether electrically active axons are preferentially myelinated by glial cells, and if so, whether axo-glial synapses are involved, are long-standing questions of significance to nervous system development, plasticity and disease. Here we show using an in vitro system that oligodendrocytes preferentially myelinate electrically active axons, but synapses from axons onto myelin-forming oligodendroglial cells are not required. Instead, vesicular release at nonsynaptic axo-glial junctions induces myelination. Axons releasing neurotransmitter from vesicles that accumulate in axon varicosities induces a local rise in cytoplasmic calcium in glial cell processes at these nonsynap-tic functional junctions, and this signalling stimulates local translation of myelin basic protein to initiate myelination.
The surprising discovery of synapses formed on glial progenitors, oligodendrocyte progenitor cells (OPCs, also called NG2 cells) has remained enigmatic for over a decade1.These cells mature to form myelin insulation on axons2,3, and several func- tions for synapses on OPCs have been proposed4.A leading hypothesis is that axon-OPC synapses may stimulate myelination selectively on electrically active axons to increase the speed of impulse transmission through electrically active neural circuits 5,6. This would have significant effects on neural circuit function. Since myelination continues in many brain regions through early life, preferential myelination of electri-cally active axons could enable environmental factors to modify neural circuit development according to functional experience7.
Synapses on OPCs could increase myelination in an activity-dependent manner in several ways,including promoting OPCs to differentiate into mature oligodendrocytes or by increasing OPC survival or proliferation. However,signals from axons must also regulate initiation of myelin wrapping even after OPCs have matured,because mature oligodendrocytes can be associated with axons early in development but not form myelin until much later in prenatal or adult life 8. It has been shown that vesicular release of glutamate from axons stimulates local translation of myelin basic protein (MBP) and stimulates myelin induction9.This signalling could be mediated by synap- tic transmission or by spillover of neurotransmitter from axo-glial synapses activating extrasynaptic glutamate receptors on OPC processes10,11.
Alternatively to synaptic transmission, other forms of axo-glial communication could signal electrical activity in axons to OPCs. Nonsynaptic release of neurotransmitter operates by both vesicular and non-vesicular release mechanisms. Neurotransmit-ters can be released in the absence of morphological synaptic contacts to activate neurotransmitter receptors on other cells (volume conduction)12. In contrast to synap-tic communication, which is a specialization for rapid (millisecond) and highly point-to-point localized signalling between axons and dendrites,volume transmission could be particularly well suited for communication between axons and myelinating glia13. Vesicle fusion is seen at axonal swellings (varicosities) that lack identifying features of a synapse. Notably missing are the close apposition of pre- and post-synaptic membranes, submembrane thickening caused by cytoskeletal proteins that organize neurotransmitter receptors and intracellular signalling molecules in the postsynaptic apparatus and the focused accumulation of synaptic vesicles docked at the presy-naptic membrane. Neurotransmitter signalling at axonal varicosities along nerve fib-res is characteristic of autonomic transmission in the enteric nervous system14 and cholinergic transmission in neocortex15, but most neurons have similar axon varico-sities. In addition to nonsynaptic vesicular release, neurotransmitters also can be released along axons through membrane channels16.
Another important question is if given a choice,will oligodendroglial cells preferential- ly myelinate electrically active axons? In addition, oligodendrocytes are multipolar cells but it is unknown how different branches of the same oligodencrocyte are instructed by axons to act autonomously and selectively synthesize myelin in those processes that are in contact with active axons. In the present experiments, calcium imaging, electron microscopy and electrophysiology were used to determine the involvement of axon-glia communication in myelination of electrically active axons in vitro. The results indicate a strong preference for oligodendrocytes to myelinate elect-rically active axons via a mechanism dependent on nonsynaptic vesicular release of glutamate but independent of synapses on OPCs.
Results
Preferential myelination of electrically active axons
The hypothesis that axons that are electrically active would be preferentially myelina-ted was tested by co-culturing OPCs with neurons that could release synaptic vesicles together with other neurons in which vesicular release was blocked. Dorsal root ganglion (DRG) neurons were used in these studies because they have several advantages. DRG neurons have no dendrites and thus they are ideal for studying oligodendrocyte interactions with axons. The long central axons of DRG neurons are myelinated by oligodendrocytes, and DRG neurons do not form synapses on them-selves (in vivo or in culture)17,18. DRG neurons do not fire action potentials sponta-neously, and they fire a single action potential in response to a brief electrical stimu-lus; thus, the firing frequency and pattern can be regulated precisely by electrical sti-mulation of neurons in cell culture.In these experiments,half of the neurons were trea- ted with the clostridial neurotoxin, botulinum A (BoNT/A) together with a blue dye to identify these axons.BoNT/A is a potent and highly selective enzyme that cleaves sy- naptosome-associated protein-25 (SNAP-25), the t-SNARE (Target membrane-asso-ciated soluble N-ethylmaleimide-sensitive factor attachment protein receptor) neces-sary for neurotransmitter release from synaptic vesicles. The other half of the neurons were untreated,providing OPCs a choice as to which axons to myelinate once under- going differentiation.After washing out the toxin,which continues to inhibit neurotrans- mitter release for at least 4 weeks19, OPCs were added to neuronal cultures contai-ning normal and BoNT/A-treated neurons to determine whether myelin formed prefe-rentially on axons that release synaptic vesicles in response to electrical stimulation (Fig. 1a,b; Supplementary Fig. 1b–d). Compact myelin was identified by immunocyto-chemistry for MBP 3 weeks after culturing OPCs on DRG axons.
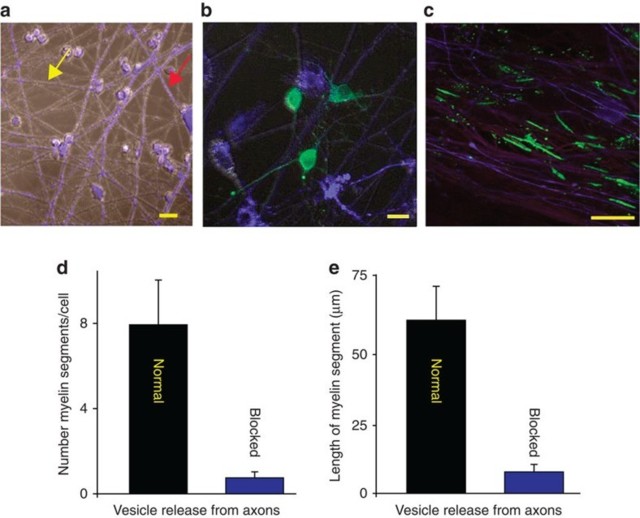
(a) DRG neurons treated with BoNT/A and stained with cell tracker (blue, see red arrow) co-cultured with normal (untreated) neurons (grey, see yellow arrow).
(b) OPCs (green, GCaMP3) were added to the cultures to determine whether exocytosis of neurotransmitter from axons influenced myelination.
(c) Axons were stimulated for 9 s at 10 Hz every 5 min for 10 h and cultured for 3 weeks. Myelin (green, myelin basic protein, MBP) analysed 3 weeks after co-culture formed preferentially on axons releasing synaptic vesicles (purple, neurofilament), and
(d) number of myelin segments/cell were more in normal axons (P<0.001, n=7 cells from four dishes)
(e) myelin segments were also longer in normal axons (P<0.001, n=9 cells from four dishes). Scale bar, 10 μm (a,b); 20 μm (c).
Consistent with the hypothesis, the results showed that when given a choice, oligo-dendrocytes preferentially myelinated axons that could release synaptic vesicles (Fig. 1c). By far, the majority of myelin segments (10 times more) were found on con-trol axons compared with axons in the same culture in which vesicular release was inhibited (Fig. 1d) (P<0.001, t-test n=7 cells from four dishes). When myelin did form on axons that were unable to release synaptic vesicles, individual myelin segments were only 1/8 as long as normal (Fig. 1e, P<0.001, t-test, n=9 cells from four dishes).
Thus,oligodendroglia preferentially myelinate electrically active axons in these expe- riments by a mechanism dependent on exocytosis. This is consistent with the hypo-thesis that synaptic transmission between axons and OPCs promotes the initial events of myelination.However,release of neurotransmitter along axons can also take place in the absence of synapses, providing an alternative explanation for this result.
Axo-glial synaptic transmission in myelination
Preferential myelination of electrically active axons could result from synaptic com-munication stimulating OPC development; however, further experimental results did not support this. Supplementary Fig. 1a shows typical OPC morphology in co-culture for 24 h with DRG neurons. In our conditions, monocultured OPCs did not exhibit in-ward sodium currents until 2–3 days in vitro (d.i.v.) (0 of 8 cells at 0–1 d.i.v. 5 of 10 at 2–3 d.i.v.; Supplementary Fig.2b,c). Interestingly,we observed that contact with axons greatly accelerated the onset of inward sodium current expression in OPCs. Sodium currents were evident in 84% of 91 recorded cells from the first day of co-culture with DRG neurons, independently of the treatment (Fig. 2a,b; Supplementary Fig. 2e). No changes on I–V curves for sodium or potassium currents, input resistance or capaci-tance of OPCs were observed in co-cultures unstimulated and pre-stimulated electrically with or without BoNT/A (Supplementary Fig. 2d–g).
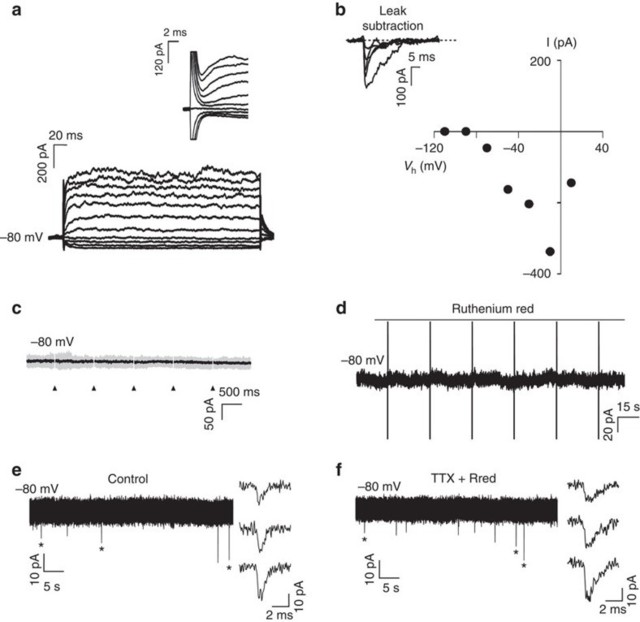
(a) Currents elicited in a recorded OPC held at −80 mV 1 day after plating by voltage steps from +20 to −110 mV. Note the presence of sodium currents (inset).
(b) I–V curve of sodium currents for the same OPC after leak subtraction (inset).
(c) Lack of evoked synaptic currents in an OPC upon DRG axonal stimulation. There was no response in 74 cells tested (unstimulated n=17 cells, from seven dishes; pre-stimulated electrically with (n=15 cells from six dishes) or without (n=42 from 17 dishes) BoNT/A). Individual (grey, 15 sweeps) and average traces (black) are shown. Axonal stimulation time is indicated with arrowheads.
(d) Bath application of the secretagogue ruthenium red (75 μM) in absence of electri-cal DRG axonal stimulation did not evoke any currents in the same OPC. Capacitive currents in response to a test pulse are shown (unstimulated n=10 cells from four dishes, pre-stimulated n=18 cells from seven dishes and pre-stimulated with BoNT/A n=7 cells from four dishes).
(e,f) Spontaneous (e) and miniature (f) synaptic currents in an OPC recorded at 15 postnatal days in acute coronal corpus callosum slices of NG2-DsRed mice (N=2 mice). Miniature synaptic currents were recorded in 1 μM tetrodotoxin (TTX) and 75 μM ruthenium red (Rred). The mean frequency, rise and decay times of spontaneous synaptic activity are 0.51 Hz, t10–90%=332 μs and τ=1.35 ms, respectively (insets, n=7 cells from seven different brain slices). The holding potential is indicated for each trace.
To test for the presence of synaptic currents in OPCs co-cultured with neurons, elect-rical stimulation (1 Hz;Supplementary Fig.2a) was applied through extracellular elect- rodes to depolarize DRG axons that traverse beneath a high-resistance barrier sepa-rating DRG cell bodies and axons in different compartments of three-compartment chambers (Campenot chambers).To test for the presence of evoked synaptic currents in OPCs cultured on axons in the central compartment, we recorded these cells in whole-cell configuration at a holding potential of −80 mV while stimulating DRG neu-rons through the extracellular electrodes. Importantly, evoked AMPAR (α-amino-3- hydroxy-5-methyl-4-isoxazole propionic acid receptor)-mediated currents in OPCs were never detected in co-cultures, regardless of whether OPCs were in contact with axons that were previously unstimulated (n=17), or axons that were pre-stimulated electrically with (n=15) or without (n=42) BoNT/A (Fig. 2c). To corroborate the lack of axon-OPC synapses, we analysed the possible existence of spontaneous synaptic currents in OPCs recorded before and during application of the secretagogue ruthe-nium red, which stimulates a massive release of synaptic vesicles. No spontaneous synaptic currents were detected in OPCs either in control conditions or in response to application of ruthenium red (unstimulated n=10; pre-stimulated n=18; pre-stimulated with BoNT/A n=7) (Fig.2d).It is noteworthy that most OPCs (80%) recorded during ex- tracellular stimulation and/or in ruthenium red had a membrane resistance >300 MΩ, sufficient for the detection of small or distant synaptic currents in these progenitors11 (SupplementaryFig.2f).In addition,confirming previous research,we find that AMPAR-mediated synaptic currents are common in recordings of OPCs in acute brain slices, using the same recording conditions that were used in co-cultures (Fig. 2e,f). Since myelination is promoted by electrical stimulation in co-cultures20,21, our results indi-cate that activity-dependent regulation of myelination does not require AMPAR-medi-ated synaptic communication between axons and OPCs. Therefore the hypothesis that synapses are required for formation of myelin on electrically active axons is not supported.
Nonsynaptic communication in activity-dependent myelination
Next,we wished to determine how OPCs could selectively myelinate axons that were electrically active in the absence of synapses from axons. DRG neurons do not form synapses in monoculture17,18; however, vesicle recycling in monoculture DRG neu-rons occurs along axons in response to electrical stimulation as shown by monitoring the fluorescent indicator FM 4–64 (Supplementary Fig.1e). Since treatment with botu- linum toxin strongly inhibited both vesicle recycling along axons induced by electrical stimulation9 and myelination independent of synaptic transmission (Figs 1 and 2), we hypothesized that nonsynaptic vesicular release of neurotransmitter along axons may stimulate myelination.
To test this possibility, we imaged cytoplasmic calcium responses in OPCs transfec-ted with the genetic calcium indicator GCaMP3 in response to electrical stimulation of axons. The results showed functional communication between axons and OPCs despite the absence of synaptic currents (Fig. 3). Ca2+ transients induced in OPCs by axonal action potentials were typically seen at points where OPC processes were associated with axons (Fig. 3, insets).
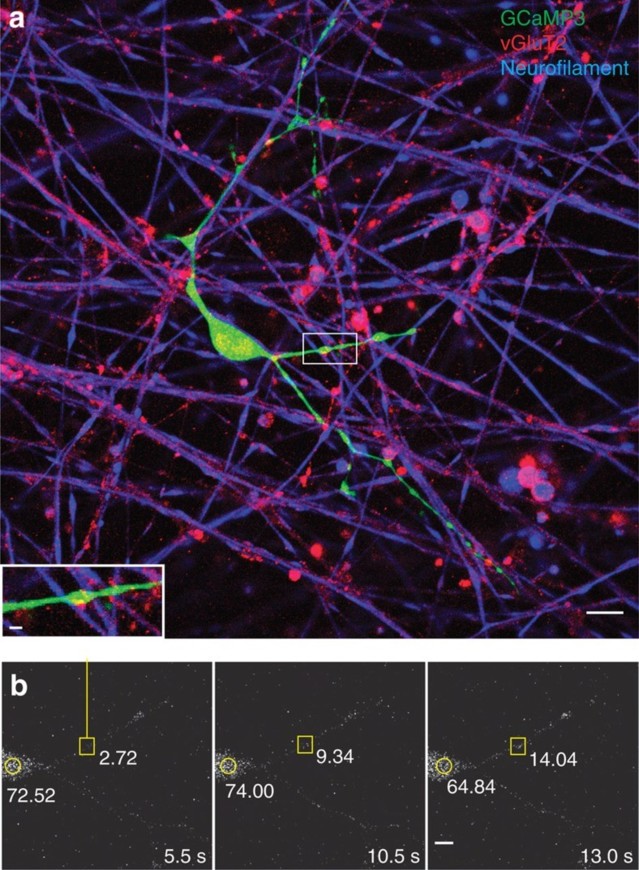
Ca2+ increases in processes of OPCs at axonal varicosities.
(a) OPC processes (green, GCaMP3 transfection) form specialized functional junc-tions with DRG axons (blue, neurofilament), containing accumulations of synaptic vesicles containing glutamate (red, vGluT2). Scale bar, 10 μm. After live-cell calcium imaging (b), the cultures were fixed and stained by immunocytochemistry to deter-mine whether that the calcium responses were associated with axo-glial contacts containing glutamatergic synaptic vesicles (inset in a). Note colocalization between axonal varicosity (red, vGluT2) and swellings in OPC process (green, GCaMP3).
Scale bar,2 μm. (b) Time-lapse series showing a local increase in Ca2+ in OPC-axon junctions in response to electrical stimulation of axons. A stimulus-induced, local in-crease in Ca2+ in the same axo-glial junction (yellow square) that is shown in a is re-corded in the OPC transfected with GCaMP3. Time since stimulus onset is shown in each frame (5.5–13 s). The fluorescence intensities at the axo-glial contact (yellow square) and the OPC cell body (yellow circle) are shown. Note the local increase in fluorescence intensity at the axo-glial junction after stimulation but this is not accompanied by an increase in fluorescence intensity in the soma. Scale bar, 5 μm.
The onset latency of electrically induced calcium rise in OPCs was much longer than synaptic transmission; >1 s. In response to pulsed stimulation (0.5 s at 10 Hz every 2 s),the average time to peak of the first response was 25±5.7 s (n=14 dishes),with mul- tiple subsequent calcium responses during stimulation sustained for 200 s (Fig. 4a, b). Application of BoNT/A significantly reduced the amplitude of electrically induced calcium response in OPCs (Fig. 4a,b). Selective inhibitors of neurotransmitter recep-tors indicate that these responses were mediated by both glutamatergic and puriner-gic signalling. A cocktail of glutamate receptor antagonists inhibiting NMDA (N- me-thyl- D-aspartate), mGluR and AMPA glutamate receptors (DAPV (D-(−)-2-amino-5- phosphonopentanoic acid),MCPG((RS)-α-methyl-4-carboxyphenylglycine) and CNQX (6-cyano-7-nitroquinoxaline-2,3-dione), respectively) reduced the amplitude of electrically evoked Ca2+ in OPCs by 80% (Fig. 4d). Suramin inhibition of purinergic receptors, or MCPG inhibition of mGluR receptors alone, reduced the amplitude of responses significantly (Fig. 4d). These results implicate vesicular release of ATP and glutamate from varicosities in signalling to OPC processes.
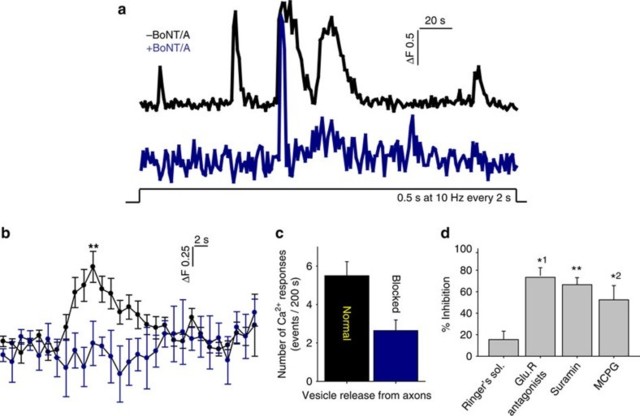
Ca2+ increase in OPC processes is mediated by glutamate and ATP released in response to action potentials in axons.
(a) Representative Ca2+ traces in OPC processes in response to electrical stimu-lation of axons without (black) and with (blue) BoNT/A treatment to block SNARE-dependent exocytosis.
(b) Averaged Ca2+ traces in OPCs from 14 dishes are shown. Responses were inhibited by BoNT/A treatment. **P=0.001, t-test, peak amplitude, n=14 cells from 14 dishes with no BoNT/A, n=14 cells from 14 dishes with BoNT/A.
(c) Number of Ca2+ responses were reduced significantly by BoNT/A treatment (blocked). The peak Ca2+ response within 200 s of axonal stimulation (10 Hz) were measured. P=0.004, t-test,n=14 cells from 14 dishes with no BoNT/A, n=14 cells from 14 dishes with BoNT/A.
(d) Summary graph shows per cent inhibition of Ca2+ responses following treatment with selective blockers of glutamatergic and purinergic neurotransmitter receptors, including a combination of CNQX, DAPV, MCPG (GluR antagonists), suramin and MCPG. The results implicate both glutamate and ATP neurotransmitter signalling. P=0.3, n=6 cells from six dishes, *1P=0.03, n=4 cells from four dishes, **P=0.008, n=5 cells from five dishes, *2P=0.02, n=6 cells from six dishes, all paired t-test.
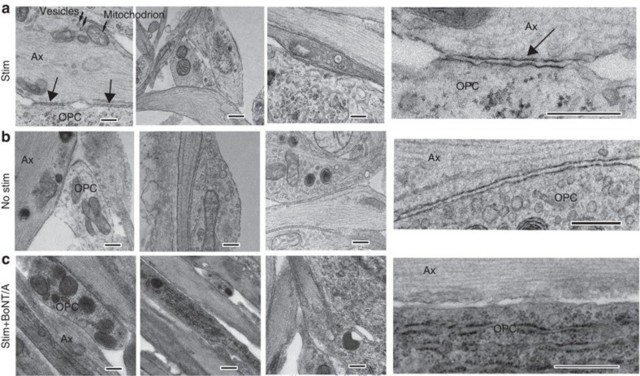
Transmission electron microscopy showed specialized contacts (arrows) between OPC processes (OPC) and axon varicosities (Ax) containing intracellular vesicles (small arrows in a) and mitochondrion, but no synapses were detected. Three examples for each condition are shown. Insets (right column) show these junctions at higher magnification. Such contacts were evident in cultures stimulated for 9 s at 10 Hz every 5 min for 10 h (Stim) before plating OPCs, and unstimulated cultures (b), but such contacts were not found in stimulated cultures treated with BoNT/A before adding OPCs (c). Scale bar, 1 μm.

OPC processes preferentially contact electrically active axons releasing synaptic vesicles and form nonsynaptic axo-glial junctions.
(a,b) Images showing OPCs transfected with GCaMP3 construct (green) and vGluT2 immunocytochemistry (red) to identify glutamate-containing vesicles in axons. OPCs were plated on axons either previously treated (b) or not treated (a) with BoNT/A. (a,b) Magnified views of glutamate-containing vesicles and OPC processes. Yellow arrows indicate vGluT2 stained puncta in close opposition to GCaMP3 processes.
(c) The number of vGluT2 puncta >0.5 μm in diameter was reduced significantly on axons previously treated with BoNT/A (P=0.0005, t-test, n=7 cells from seven dishes with no BoNT/A, n=8 cells from eight dishes with BoNT/A).
(d,e) Images showing OPC transfected with GCaMP3 construct (green) and immuno-cytochemistry for neurofilament (blue, an axon marker). (f) Summary graph showing that the fraction of all OPC processes (d,e) in individual OPCs forming parallel asso-ciations with axons was significantly reduced when axons were previously treated with BoNT/A (P=0.0002,t-test, n=7 cells from seven dishes with no BoNT/A, n=8 cells from eight dishes with BoNT/A). Scale bars, 10 μm (a, b,d,e); 2 μm (a,b).
Interestingly, OPC processes were less closely associated with axons in which exocytosis was inhibited by BoNT/A treatment. Rather than adhering to the axon and tracking along it, OPC processes frequently intersected and crossed over axons rather than running parallel together with the axon (Fig. 6d–f; P=0.0002, t-test, n=15 cells from 15 dishes).
Formation of specialized junctions between axons and oligodendrocytes, which were described as ‘spot welds' in the earliest electron microscopic study of central nervous system myelination,is the first event in myelin formation23.Importantly,these junctions shown here in cell culture and previously in vivo23,24 lack ultrastructural specializa-tions characteristic of synaptic junctions. In line with these anatomical observations, our findings show that specialized nonsynaptic axon-OPC junctions are functional and signal via vesicular release from axon varicosities that induces Ca2+ increases in OPCs and may serve to promote myelination.
Autonomous oligodendrocyte processes in activity-dependent myelination
Oligodendrocytes are multipolar cells that can myelinate up to 50 different axons. Axonal factors are known to induce myelination, but it is unclear whether individual cell processes of an oligodendrocyte can act autonomously to myelinate different axons, or whether oligodendrocyte commitment to myelin induction is a cell-wide event. Local translation of myelin basic protein is stimulated in OPCs by glutamate released from axons acting on mGluR and NMDA receptors on oligodendrocytes9 but it is unknown whether this occurs selectively in those processes of an OPC that are in contact with electrically active axons. To answer this question, OPCs were transfected with kikume MBP-3′-untranslated region, a photo-activated fluorophore that enables identification of newly synthesized protein by a change in fluorescence from red to green after photoactivation. Local translation of MBP was found to be pre-ferentially induced in OPC processes in contact with electrically active axons compa-red with OPC processes from the same cell in contact with axons in which vesicular release had been inhibited by prior treatment with BoNT/A (Fig 7). Thus, different cell processes of an oligodendrocyte act independently in myelin induction and thus are able to compartmentalize signals.We conclude that one of the axonal factors determi- ning whether different branches of an oligodendrocyte myelinate an axon is the activity-dependent exocytosis of vesicles containing neurotransmitter along the fibre.
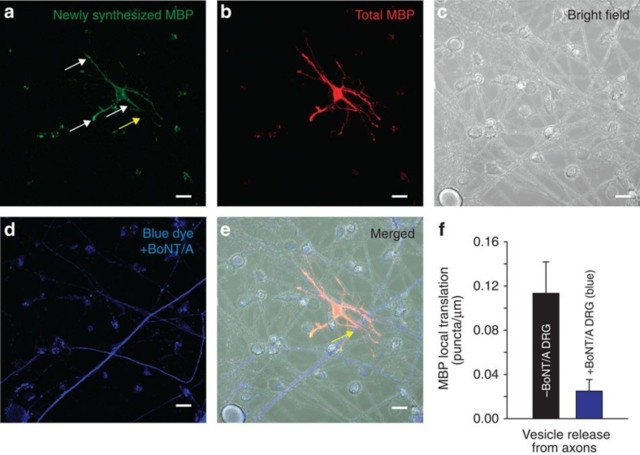
Local translation of MBP occurs preferentially on OPC processes contacting electrically active axons releasing synaptic vesicles.
(a) Newly synthesized MBP (green). Axons were stimulated electrically at 10 Hz for 10 min and local translation of MBP was monitored using kikume MBP fluorescence after 40 min. White arrows indicate new MBP translation in contrast yellow arrow showing no new MBP translation.
(b) Total MBP (red).
(c) Bright field showing cell morphology of co-culture.
(d) Axons treated previously with BoNT/A to block synaptic vesicle release (blue).
(e) Combined images from a–d.
Differential interference contrast and fluorescence to identify axons treated (blue, yel-low arrow) and not treated with BoNT/A. Yellow arrow shows axon and OPC interac-tion without MBP translation (notice yellow arrow in a with lack of new MBP green puncta indicating no new MBP translation).
(f) Quantification shows significantly more local translation of MBP in OPC processes that were in contact with axons releasing synaptic vesicles (grey axons) (0.11±0.024 versus 0.028±0.011 puncta per micrometre length of axon not treated or previously treated with BoNT/A respectively, P<0.001, t-test, n=24 cells from non-blue, n=23 cells from blue axons, five dishes). Scale bar, 10 μm.
Discussion
The experiments provide several novel findings: When provided a choice, OPCs pre-ferentially myelinate electrically active axons releasing synaptic vesicles. This activi-ty-dependent communication between axons and OPCs is mediated by nonsynaptic intercellular junctions that signal through intracellular calcium. Myelin forms normally and is stimulated by electrical activity in axons in culture even though there is no evi-dence of synapses by electrophysiological and electron microscopic analysis. Indivi-dual cell processes of oligodendrocytes act autonomously, initiating myelination in response to electrical activity in axons in contact with the oligodendroglial process, which stimulates local translation of MBP in that subcellular domain.
In conclusion, myelin is formed preferentially on electrically active axons releasing vesicles, but synapses on OPCs are not required for activity-dependent stimulation of myelination. Local calcium signalling produced by vesicular release of glutamate is well suited to local subcellular control of myelin induction in individual OPC proces-ses, by activating glutamate receptors localized in the fine processes closely associ-ated with axons9. ATP release can also occur from synapses and mediate discrete signals, but purinergic signalling appears to be more relevant to modulating differen-tiation and proliferation of OPCs, in part because these signalling pathways activate global intracellular calcium responses in the cell9. This is possibly associated with wider spread release of ATP from axons through volume-regulated anion channels 16, and differences in localization of glutamate and purinergic receptors in the cell.
Preferential myelin induction on electrically active axons would have profound effects on circuit function by the resulting increased conduction velocity,and thus provide another mechanism of plasticity complementing synaptic plasticity7. In human brain imaging studies, white matter structure is affected by learning25, and recent studies show that social isolation impairs myelin formation in the forebrain of mice 26,27; the present findings could provide a cellular mechanism participating in the effects of en-vironmental experience on myelination. These new findings may also have implica-tions for disease, including psychiatric illness and impaired remyelination after conduction block in multiple sclerosis.
Methods
Cell culture
All experiments were conducted in accordance with animal study protocols approved by the NICHD Animal Care and Use Committee. DRG neurons were dissected from the spinal cords of embryonic day-13.5 mice as described9 Neurons were grown for ∼ 4 weeks in MEM media supplemented with N3 containing 100 ng ml−1 of nerve growth factor and 5% heat-inactivated horse serum in the side compartments of three-compartment chambers equipped with stimulating electrodes28 or on cover-slips that were coated with poly-L-lysine and collagen. Mitosis of non-neuronal cells was inhibited by a 4-day treatment with 13 μg ml−1 fluoro-2′-deoxyuridine beginning 1 day after plating. These cultures can be maintained indefinitely with half-volume changes of media every 3 days. Primary cultures of OPCs were obtained from cereb-ral cortices of embryonic day-19 P2 rats and plated into 75-cm2 tissue culture flasks.
The resulting cultures were maintained at 5% CO2 and 37 °C in media containing 10% fetal bovine serum (Life Technologies, Carlsbad,CA, USA). After 11 days in cul- ture, the flasks were shaken at 37 °C for 1 h to kill non-glial cells and remove micro-glia, then the media was changed and the flasks were shaken overnight to lift OPCs from the flask.To enrich for OPCs,the cell suspension was pelleted,resuspended and incubated in an uncoated culture dish for 30 min.Contaminating cells, primarily endo- thelial cells, astrocytes,macrophages and microglia adhere strongly to the plastic and can be separated out by this panning method. For myelinated co-cultures, purified OPCs (>90% OPCs) were counted and plated on 3–4-week-old DRG cultures at a density of 40,000 cells per side compartment.After 3–4 h,the media was removed and replaced with N1 differentiating media. Action potentials were induced in DRG axons by a 200-μs 5-V biphasic pulse through platinum electrodes in three-compartment chambers28. Axons growing into the central compartment beneath the high-resistant barriers are stimulated but cells in the lateral compartments are not depolarized. For 3-week co-culturing myelination experiments, stimulation was applied in 0.5 s bursts at 10 Hz every 2 and 15 s bursts at 10 Hz every 5 min, using biphasic pulses (200-μs 6 V, square-wave pulse) for 5 h (ref. 9). For Ca2+ imaging, field stimulation (15 s at 10 Hz, 30–50 V) was delivered through two 1-cm-long horizontal platinum electrodes in cultures of DRG neurons grown on 25-mm coverslips.
Intracellular Ca2+ imaging
OPCs transfected with GCaMP3 were imaged during electrical stimulation using a confocal microscope equipped with a 40 × (1.3 numerical aperture, NA) objective lens, excitation at 488 nm by scanning laser, and emission light filtered through a HQ528/50 splitter/filter. Quantification of images was performed using Image J software (NIH).
Vesicle recycling
Recycling synaptic vesicles in DRG axons were labelled with FM 4–64 (Life Techno-logies). To stain the total pool of recycling vesicles, axons were loaded by electrical stimulation (10 Hz,30 V, 5 ms, biphasic square wave) for 20 s in media containing 2.5 μM FM 4–64 dye. The dye was allowed to remain on the cells for 60 s after cessation of the stimulus to permit complete compensatory endocytosis, and was subsequently removed during a 7-min period with eight complete solution changes.
Pharmacology
Drugs were added directly to the culture dish before experiments. Botulinum toxin A, kindly provided by E. A. Johnson, was added to cultures at a final concentration of 3 nM for at least 18 h before experiments to block vesicular release19. Immunoblotting confirmed cleavage of SNAP-25 in DRG neurons treated with BoNT/A. Previous stu-dies show that block of neurosecretion from DRG neurons in cell culture occurs with-in 4 h of BoNT/A and last at least 4 weeks19. MCPG (500 μM), DAPV (50 μM), CNQX (20 μM), and suramin (50 μM) were obtained from Tocris (Ellisville, MO, USA). Cell Tracker Blue CMAC Dye is from Life Technologies (NY, USA).
Confocal microscopy
All images were acquired on a Zeiss 510 NLO confocal microscope (Carl Zeiss MicroImaging, Inc. Thornwood, NY,USA) equipped with both 40 × (1.3 NA) and 63 × (1.4 NA) oil-immersion lenses using appropriate laser lines and excitation/emission filters. For live-cell imaging, coverslips were mounted in an imaging chamber and continuously superfused with sterile-filtered saline. Photoactivation of Kikume was performed with ultraviolet lamp exposure for >10 min.
Antibodies
The antibodies used were as follows: vGluT2 (1:1,000, Cat. AB2251, Millipore, Bille-rica, MA, USA), neurofilament (1:5,000, Cat. NFH, Aves Labs, Tigard, Oregon), MBP (1:1,000, Cat. SMI-99, Sternberger Monoclonals), synaptophysin 1 (1:1,000, Cat. 101 011, Synaptic Systems, Gottingen, Germany), Olig2 (1:250,Cat. 18953, IBL Co., LTD, Japan) and NG2 chondroitin sulfate proteoglycan (1:500, Cat. MAB5384, Chemicon International, Billerica, MA, USA).
Electron microscopy
Cell cultures were fixed with 2% paraformaldehyde/2% glutaraldehyde in 0.13 M sodium cacodylate buffer, pH 7.4 at 37 °C and postfixed in 2% OsO4 for 2 h at 4 °C. Samples were dehydrated in a graded series of alcohol, infiltrated in propylene oxide / Spurr's and embedded in Spurr/s resin. Ultrathin sections were cut with a diamond knife, stained with uranyl acetate and lead citrate and examined by transmission electron microscopy.
Electrophysiological recordings
Patch-clamp recordings of OPCs from monocultures or DRG-OPC co-cultures were performed in voltage-clamp mode 1–3 days after plating at room temperature and using an extracellular solution containing (in mM):126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 20 glucose, 5 pyruvate, 2 CaCl2 and 1 MgCl2 (95% O2 and 5% CO2). The intracellular solution contained (in mM): 120 K-gluconate, 5 NaCl, 3 MgCl2, 0.2 EGTA, 10 HEPES, 0.3 Na-GTP,4 Na-ATP and 10 Na-phosphocreatine (pH≈7.3, 295 mOsm). To investigate the presence of fast inward AMPAR-mediated synaptic currents in OPCs of DRG-OPC co-cultures, DRG axons were stimulated while OPCs were recorded in voltage-clamp mode at a holding potential of −80 mV in co-cultures previously unstimulated and pre-stimulated electrically with or without BoNT/A. Hol-ding potentials were corrected by a junction potential of −10 mV. Extracellular stimu-lation of DRG axons was performed using a bipolar electrode placed in a side com-partment (0.5–5 V, 1–10 ms of duration). Single pulses or train of stimuli at a rate of 1, 10 and 100 Hz were applied for each neuron (Supplementary Fig. 2a).
Recordings were made without series resistance compensation. Series resistances were monitored during recordings and cells showing a change of >30% were discar-ded. Whole-cell recordings were obtained using Multiclamp 200B amplifier (Molecu-lar Devices),filtered at 5 kHz and digitized at 10 kHz.Digitized data were analysed off-line using pClamp 10.1 software (Molecular Devices). Current densities for steady-state I–V relationships of OPCs were obtained by dividing the measured current am-plitudes by cell capacitance. The amplitudes of inward sodium currents were mea-sured at different voltage steps from +20 to −110 mV after leak subtraction using a MATLAB custom routine (MATLAB, MathWorks, Inc). The ohmic leak current for each potential was estimated by scaling the responses at most hyperpolarizing pulses. Means and data distributions of steady-state and inward sodium current densities, membrane resistance and capacitance were not different among treatments (P>0.05) (Supplementary Fig. 2d–g).
Statistical analysis
Statistical significance was tested by analysis of variance in experimental designs in-volving multiple groups, followed by Dunnet's post hoc test for evaluating differences with respect to a control group and Fischer's comparison test for designs comparing differences among all groups. Two-sided Student's t-test was used for analysis of experimental designs with two groups, and a paired t-test was used in experiments in which repeated measures were made in the same sample. Data are displayed as means and s.e.m. For electrophysiological data, each data group was first subject to D'Agostino and Pearson normality test. According to the data structure (non-normally or normally distributed), the Kruskal–Wallis or two-way analysis of variance test was used for comparisons. Multiple Kolmogorov–Smirnov test was used to compare distributions of data. All statistical analysis and plotting were performed with Minitab version 12 (State College, PA, USA), SigmaPlot and GraphPad Prism 5.00 software (GraphPad Software Inc., USA).
Additional information
How to cite this article: Wake, H. et al. Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat. Commun. 6:7844 doi: 10.1038/ncomms8844 (2015).
Supplementary Material
Acknowledgments
We thank Andrea Helo for her help on building the MATLAB custom routine used for leak subtraction. This work was supported by NICHD funds for intramural research. M.C.A. is part of the ENP-Ile-de-France network and was supported by grants from Agence Nationale de la Recherche (ANR, R14193KK), Fondation pour l'aide à la recherche sur la Sclérose en Plaques (ARSEP) and IDEX-Cité Paris Sorbonne. FCO is supported by a post-doc fellowship from Fondation pour la Recherche Médicale (FRM).
Footnotes
Author contributions All authors contributed to the data analysis,experimental plan-ning and writing the paper. R.D.F. ideated and directed the project. H.W., D.H.W. and P.R.L. performed the non-electrophysiological experiments and F.C.O. performed the electrophysiological recordings.
References
- Bergles D. E., Roberts J. D., Somogyi P. & Jahr C. E. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405,187–191 (2000). [PubMed] [Google Scholar]
- Emery B. Regulation of oligodendrocyte differentiation and myelination. Science 330, 779–782 (2010). [PubMed] [Google Scholar]
- Fields R. D. Chapter in Neuroglia 3rd edn eds Kettenmann H., Ransom B. R. 573 – 585Oxford Univ. Press (2013). [Google Scholar]
- Mangin J. M. & Gallo V. The curious case of NG2 cells: transient trend or game changer? ASN Neuro 3, e00052 (2011). [PMC free article] [PubMed] [Google Scholar]
- Ziskin J. L., Nishiyama A., Rubio M., Fukaya M. & Bergles D. E. Vesicular release of glutamate from unmyelinated axons in white matter. Nat.Neurosci.10,321 – 330 (2007). [PMC free article] [PubMed] [Google Scholar]
- Kukley M.,Capetillo-Zarate E.&Dietrich D.Vesicular glutamate release from axons in white matter. Nat. Neurosci. 10, 311–320 (2007). [PubMed] [Google Scholar]
- Fields R. D. Neuroscience. Change in the brain's white matter. Science 330, 768 – 769 (2010). [PMC free article] [PubMed] [Google Scholar]
- Back S. a., Luo N. L., Borenstein N. S., Volpe J. J. & Kinney H. C. Arrested oligo-dendrocyte lineage progression during human cerebral white matter develop-ment: dissociation between the timing of progenitor differentiation and myelinoge-nesis. J. Neuropath. Exp. Neurol. 61, 197–211 (2002). [PubMed] [Google Scholar]
- Wake H., Lee P. R. & Fields R. D. Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647–1651 (2011). [PMC free article] [PubMed] [Google Scholar]
- Maldonado P. P. & Angulo M. C. Multiple modes of communication between neurons and oligodendrocyte precursor cells. Neuroscientist 21, 266–276 (2015). [PubMed] [Google Scholar]
- Velez-Fort M., Maldonado P. P., Butt A. M., Audinat E. & Angulo M. C. Postnatal switch from synaptic to extrasynaptic transmission between interneurons and NG2 cells. J. Neurosci. 30, 6921–6929 (2010). [PMC free article] [PubMed] [Google Scholar]
- Vargova L. & Sykova E. Extracellular space diffusion and extrasynaptic transmission. Physiol. Res. 57, S89–S99 (2008). [PubMed] [Google Scholar]
- Fields R. D. Volume transmission in activity-dependent regulation of myelinating glia. Neurochem. Int. 45, 503–509 (2004). [PubMed] [Google Scholar]
- Burnstock G. Non-synaptic transmission at autonomic neuroeffector junctions. Neurochem. Int. 52, 14–25 (2008). [PubMed] [Google Scholar]
- Zhang Z. W., Kang J. I. & Vaucher E. Axonal varicosity density as an index of local neuronal interactions. PLoS ONE 6, e22543 (2011). [PMC free article] [PubMed] [Google Scholar]
- Fields R. D. & Ni Y. Nonsynaptic communication through ATP release from volume-activated anion channels in axons. Sci. Signal. 3, ra73 (2010). [PMC free article] [PubMed] [Google Scholar]
- Ransom B. R., Neale E., Henkart M., Bullock P. N. & Nelson P. G. Mouse spinal cord in cell culture. I. Morphology and intrinsic neuronal electrophysiologic properties. J. Neurophysiol. 40, 1132–1150 (1977). [PubMed] [Google Scholar]
- Ransom B. R., Christian C. N., Bullock P. N. & Nelson P. G. Mouse spinal cord in cell culture. II. Synaptic activity and circuit behavior. J. Neurophysiol. 40, 1151 – 1162 (1977). [PubMed] [Google Scholar]
- Welch M. J., Purkiss J. R. & Foster K. A. Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon 38, 245–258 (2000). [PubMed] [Google Scholar]
- Stevens B., Porta S., Haak L.L., Gallo V. & Fields R. D. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron 36, 855–868 (2002). [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T. et al. Astrocytes promote myelination in response to electrical impul-ses. Neuron 49, 823–832 (2006). [PMC free article] [PubMed] [Google Scholar]
- Peters A. & Palay S. L. The morphology of synapses. J. Neurocytol. 25, 687–700 (1996). [PubMed] [Google Scholar]
- Luse S. A. The fine structure of the morphogenesis of myelin. Prog. Neurobiol. 4, 59–95 (1959). [PubMed] [Google Scholar]
- Alix J.J.,Dolphin A.C. & Fern R. Vesicular apparatus, including functional calcium channels, are present in developing rodent optic nerve axons and are required for normal node of Ranvier formation. J. Physiol. 586, 4069–4089 (2008). [PMC free article] [PubMed] [Google Scholar]
- Zatorre R. J., Fields R. D. & Johansen-Berg H. Plasticity in gray and white: neuro-imaging changes in brain structure during learning. Nat. Neurosci. 15, 528–536 (2012). [PMC free article] [PubMed] [Google Scholar]
- Makinodan M., Rosen K. M., Ito S. & Corfas G. A critical period for social experi-ence–dependent oligodendrocyte maturation and myelination. Science 337, 1357 – 1360 (2010). [PMC free article] [PubMed] [Google Scholar]
- Liu J. et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 15, 1621–1623 (2012). [PMC free article] [PubMed] [Google Scholar]
- Fields R. D., Yu C., Neale E. A. & Nelson P. G. In Electrophysiological Methods for In Vitro Studies in Vertebrate Neurobiology eds Kettenmann H., Grantyn R. 67–76Alan R. Liss (1992). [Google Scholar]
Tässä on pääpaino mekanismin muodostumisella mm. geneettisesti eikäm eikä sen toiminnalla:
" The Brain Prize 2023
Pioneering work on molecular mechanisms of brain development and plasticity awarded with the world’s top prize in neuroscience
Professors Michael Greenberg, Christine Holt and Erin Schuman have revolutionized our understanding of how neurons regulate the thousands of different proteins – the building blocks of life, that are needed to support brain development, plasticity and maintenance. They have revealed crucial molecular mechanisms that sustain the development and function of the healthy brain and also provided key insights into the causes of neurodevelopmental and neurodegenerative diseases.

Copenhagen, Denmark - The Lundbeck Foundation has announced the recipients of The Brain Prize 2023, the world’s largest award for outstanding contributions to neuroscience. This year’s award recognizes the pioneering work of three leading neuroscientists - Professor Michael Greenberg at Harvard Medical School, Professor Christine Holt at University of Cambridge, and Professor Erin Schuman at the Max Planck Institute for Brain Research.
The Brain Prize 2023 worth DKK 10 million (€1.3 million) is awarded to:
- Christine Holt (UK)
- Michael Greenberg (USA)
- Erin Schuman (Germany)
A profound aspect of our nervous system is that during development and adulthood our brains are subject to extensive change, known as neural plasticity. Such plasticity requires that the complement of neural proteins - the neural proteome, be dynamically regulated in space and time. An international group of three neuroscientists, Michael Greenberg, Christine Holt, and Erin Schuman have each revealed the fundamental principles of how this is mediated at the molecular level – from activity-dependent gene transcription to the local translation of mRNA into new proteins in dendrites and growing axons.
Their findings have provided spectacular new insights into the cellular and molecular mechanisms that guide growing axons during brain development, and that enable the developing and adult brain to be shaped by experience. Theirs is a beautiful discovery story in fundamental neuroscience that also provides clues to the aetiology of neurodevelopmental and neurodegenerative diseases of the brain. For their work, the three neuroscientists are awarded the world’s largest prize for brain research – The Brain Prize.
Professor Richard Morris, Chair of The Brain Prize Selection Committee explains the reasoning behind this year’s award.
The Brain Prize winners of 2023, Michael Greenberg, Christine Holt, and Erin Schuman have revealed the fundamental principles of how this enigmatic feature of brain function is mediated at the molecular level. Together, the Brain Prize 2023 winners have made ground-breaking discoveries by showing how the synthesis of new proteins is triggered in different neuronal compartments, thereby guiding brain development and plasticity in ways that impact our behavior for a lifetime.’’
“On behalf of the Lundbeck Foundation, I am delighted that The Brain Prize 2023 is awarded to these three outstanding neurobiologists,” said Lene Skole, CEO of the Lundbeck Foundation.
“Their pioneering research has broken new ground and provided deep insights into the molecular mechanisms of neural development and plasticity. Their work also provides vital new insights into the causes and mechanisms of some of the most devastating disorders of the brain. The awarding of this year’s Brain Prize is thoroughly well-deserved.”
About the Brain Prize
The Brain Prize is the world’s largest neuroscience research prize, and it is awarded each year by the Lundbeck Foundation. The Brain Prize recognises highly original and influential advances in any area of brain research, from basic neuroscience to applied clinical research. Recipients of The Brain Prize may be of any nationality and work in any country in the world. Since it was first awarded in 2011 The Brain Prize has been awarded to 44 scientists from 9 countries.
Brain Prize recipients are presented with their award by His Royal Highness, The Crown Prince of Denmark, at a ceremony in the Danish capital, Copenhagen.
About the Lundbeck Foundation
The Lundbeck Foundation is an enterprise foundation encompassing a comprehensive range of commercial and philanthropic activities – all united by its strong purpose; Bringing Discoveries to Lives. The Foundation is the long-term and engaged owner of several international healthcare companies – Lundbeck, Falck , ALK, and Ferrosan Medical Devices – and an active investor in business, science and people through its commercial investments in the financial markets; in biotech companies based on Danish research and through philanthropic grants to science talents and programmes in Danish universities. The Foundation’s philanthropic grants amount to more than DKK 500m annually primarily focusing on the brain – including the world’s largest personal prize awarded in neuroscience, The Brain Prize. "


Kommentit