http://europepmc.org/backend/ptpmcrender.fcgi?accid=PMC4426493&blobtype=pdf
HHS Public Access
Author manuscript
Neuron. Author manuscript; available in PMC 2016 April 22.
s. 374
Glial Regulation of the Neuronal Connectome through Local and Long-Distant Communication
R. Douglas Fields, 1, * Dong Ho Woo, 1 and Peter J. Basser 2
1 Nervous System Development and Plasticity Section, The Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD 20892, USA
2 Section on Tissue Biophysics and Biomimetics, Program on Pediatric Imaging and Tissue Sciences, The Eunice Kennedy Shriver National
Institute of Child Health and Human Development, NIH, Bethesda, MD 20892, USA
*Correspondence: [email protected]
http://dx.doi.org/10.1016/j.neuron.2015.01.014
If ‘‘the connectome’’ represents a complete map of anatomical and functional connectivity in the brain, it should also include glia. Glia define and regulate both the brain’s anatomical and functional connectivity over a broad range of length scales, spanning the whole brain to subcellular domains of synaptic interactions. This Per-spective article examines glial interactions with the neuronal connectome (including long-range networks, local circuits, and individual synaptic connections) and highlights opportunities for future research. Our understanding of the structure and function of the neuronal connectome would be incomplete without an understanding of how all types of glia contribute to neuronal connectivity and function, from single synapses to circuits.
Introduction
Astrocytes, microglia, and oligodendrocytes all have biological properties that influ-ence neural connectivity, both structurally and functionally. All types of glia can res-pond to and influence neurotransmission in several ways; thus, glia are as pertinent to the brain’s connectome as any neuron. Glia are very different from neurons (Ket-tenman and Ransom,2013) and contribute to information processing and connectivi-ty differently from neurons. Glia differ from neurons in terms of morphology, signaling mechanisms, and the spatial and temporal scales at which they operate, but glia share a common neurotransmitter-based signaling system with neurons (Parpura et al.,1994; Nedergaard, 1994), thus incorporating them into a mutual system.
Moreover, neurons are dependent on glia for metabolic support (Brown et al., 2003; Funfschilling et al., 2012; Hirrlinger and Nave, 2014), regulation of extra-cellular ion concentration necessary for neuronal excitability (Pannasch et al.,2011), local regu-lation of blood flow to small populations of neurons engaged in a functional operation (Mulligan and MacVicar, 2004), and glia release growth factors, cytokines, and other neuromodulatory molecules that regulate neuron structure, function,and connectivity, for example brain-derived neurotrophic factor (BDNF) (Gómez-Casati et al., 2010), cholesterol (Mauch et al., 2001), Ephrin (Murai et al., 2003), TGF beta (Diniz et al., 2012), and TNF alpha (Stellwagen and Malenka, 2006).
Glia help determine and modulate the physical structure of the neuronal connec-tome.Astrocytes form barriers to neuronal connectivity (Cooper and Steindler, 1986), guide neurite outgrowth (Kanemaru et al.,2007), modulate volume transmission with- in the extracellular space (Nicholson and Syková,1998), and moderate structural dy-namics of dendritic spines (Nishida and Okabe,2007). Astrocytes stimulate synapto-genesis (Eroglu et al., 2009; Allen et al., 2012) and remove synapses (Tasdemir-Yilmaz and Freeman, 2014).
Microglia can also remove synapses in an activity-dependent manner, thereby alte-ring connectivity in neural networks (Wake et al.,2009; Paolicelli et al., 2011; Schafer et al., 2012). Astrocytes produce growth factors that modulate neural stem cell differentiation (Barkho et al., 2006) and thus regulate neurogenesis.
Oligodendrocyte progenitor cells (OPCs or NG2 glia) are the largest population of di-viding cells in the adult brain, suggesting a possible role in myelination in the mature brain (Young et al., 2013) that could contribute to optimal information processing through long-range networks (Fields,2008). Myelination by oligodendrocytes also determines the patterns of neuronal connectivity by inhibitory proteins in myelin (Schmandke et al., 2014) that limit axonal sprouting and thereby restrict the patterns of synaptic connectivity (Fields,2014).This partitioning is obvious even by a rudimen- tary examination of the gross anatomy of the brain, which is divided into domains of gray and white matter by oligodendrocytes.
The ultimate goal of connectomics is to obtain an understanding of the functional organization and operation of neural networks.This cannot be achieved solely by tra- cing the pathways of connections between neurons. Resting state functional magne-tic resonance imaging (fMRI) of people born without a corpus callosum connecting the left and right hemispheres are remarkably normal despite the absence of the lar-gest white matter bundle in the brain (Owen et al.,2013).The brain is highly intercon- nected, such that multiple alternative routes through the anatomical connectome are possible to yield the same functional network. Glia modify neuronal connectivity by producing structural changes in the neuronal connectome, but glia also influence the functional connectome by modifying the flow of information through neural networks. Astrocytes modulate synaptic function (Panatier et al.,2006;Perea and Araque,2007) and therefore modulate neuronal connectivity through effects on neuro-transmission, but glia also coordinate and regulate neuronal functional connectivity by many other mechanisms, which will be discussed.
375

Figure 1. Conduction Time between Relay Points in Neural Circuits Must Be Precise for Spike-Timing-Dependent Plasticity and Sustaining Oscillations over Long-Dis-tance Networks Myelin is the most effective means of increasing conduction velocity; thus myelin strongly influences network function and by activity-dependent feedback, may contribute to nervous system plasticity.
(A) Mouse optic nerve reconstructed from several hundred ultra-thin sections obtained by serial block-face electron microscopy. Such new methods are enabling network analysis of myelination at an ultrastructural level.
(B) Optic nerve in cross section analyzed by transmission electron microscopy. Note the multiple layers of compact membrane (myelin) wrapped around axons.
(C) Three-dimensional reconstruction of the node of Ranvier from serial block-face electron microscopy, as shown in (A). Note the compact myelin (purple) wrapped around the axon (gray), forming a spiral channel of cytoplasm appearing as a series of paranodal loops flanking the node (gold). The electrogenic node of Ranvier, where voltage-gated sodium channels are concentrated, is ensheathed by perinodal astrocytes (blue). Scale bar, 10 mm in (A), 1 mm in (B) and (C).
375
Transmission of action potentials through myelinated axons is entirely dependent on glial cells that form the myelin sheath, and thus oligodendrocytes profoundly affect the functional connectome of neurons. By modulating conduction time, oligodendro-cytes may provide an important source of adaptive modulation for optimal information processing in complex neural networks (Fields, 2005).
Glia are interconnected through multiple mechanisms. Astrocytes are coupled through gap junctions (Giaume et al., 1991) and also by neurotransmitter-based signaling that enables selective communication to other astrocytes that have the appropriate membrane receptors (Sul et al., 2004). Oligodendrocytes can also be coupled to astrocytes through gap junctions (Maglione et al., 2010; Nualart-Marti et al., 2013). Microglia, astrocytes,and oligodendrocytes all interact with each other and with neurons via contact-mediated and diffusible signaling molecules. In contrast to the neuronal connectome,very little is known about the structure and function of glial assemblies. Connectomics includes long-range networks,local circuits,and individual synaptic connections. Glia impact all three scales of neuronal connections and each of these will be considered separately.
Long-Range Connectivity Conduction Time
A fundamental feature of neural coding, dendritic integration, and synaptic plasticity is the temporal coincidence of action potentials converging upon a neuron. Thus, the physiological mechanisms underlying information processing and learning will be strongly influenced by the conduction time through each link in the structural connec- tome. In animals with large brains, and especially in complex cognitive tasks that re-quire relaying information through multiple cortical and subcortical networks, conduc- tion delays are considerable - hundreds of milliseconds,while neural coding, dendri-tic integration, and synaptic plasticity are dependent on spike time arrival with a high degree of precision falling within a narrow range of a few milliseconds (Mu and Poo, 2006). Conduction velocities in neural circuits range widely from #200 m/s to frac-tions of a m/s to achieve the appropriate conduction delay required for each circuit to function properly and to interact appropriately with other circuits (Stanford, 1987). How the correct conduction delay is established in every link of the brain’s connec-tome and whether conduction delays can be regulated to optimize information pro-cessing and learning are critical questions at the forefront of neurobiology research.
Evidence suggests that glia have a central role in determining and possibly adaptive-ly modifying conduction delays through individual links in the neuronal connectome. In vertebrates, myelin is the most effective means of speeding conduction velocity by fundamentally changing the way action potentials propagate (via saltatory conduction) (Figure 1).
Oligodendrocytes form the myelin sheath on axons, and thus these glial cells will have a profound influence on the functional conbrains, and especially in complex cognitive tasks that require relaying information through multiple cortical and subcor-tical networks, conduction delays are considerable - hundreds of milliseconds, while neural coding, dendritic integration, and synaptic plasticity are dependent on spike time arrival with a high degree of precision falling within a narrow range of a few mil-liseconds (Mu and Poo, 2006). Conduction velocities in neural circuits range widely from #200 m/s to fractions of a m/s to achieve the appropriate conduction delay required for each circuit to function properly and to interact appropriately with other circuits (Stanford, 1987).
How the correct conduction delay is established in every link of the brain’s connec-tome and whether conduction delays can be regulated to optimize information pro-cessing and learning are critical questions at the forefront of neurobiology research. Evidence suggests that glia have acentral role in determining and possibly adaptive-ly modifying conduction delays through individual links in the neuronal connectome.
In vertebrates,myelin is the most effective means of speeding conduction velocity by fundamentally changing the way action potentials propagate (via saltatory conduc-tion) (Figure 1). Oligodendrocytes form the myelin sheath on axons, and thus these glial cells will have a profound influence on the functional conwill have a profound effect on information processing and functional connectivity.
s. 376
Activity-Dependent Myelination
Myelination proceeds postnatally in most brain regions and in some regions, such as the prefrontal cortex, myelination continues actively into the third decade of life (Ya-kovlev and Lecours, 1967). This postnatal period of myelination provides the oppor-tunity for environment and experience to modulate heredity in directing development of neuronal connections. Much less is known of experience-dependent mechanisms of glial regulation of neuronal connectivity in comparison to the large volume of infor-mation on activity-dependent synaptic plasticity. However, changes in the functional connectome through early life and adolescence depend heavily on myelination as is evident from electroencephalogram (EEG) (Smit et al.,2012) and MRI studies (Dean et al., 2014).
Even subtle changes in myelin structure will have large effects on conduction delays, refractory eriod, spike firing frequency, and spike arrival time (Pajevic et al., 2014). In mapping the structural connectome, it will be essential to define the myelin structure of axons. That is,whether or not the axon or fiber tract is myelinated,and if it is myeli- nated, what is the thickness of the myelin sheath relative to the axon diameter and how are the nodes of Ranvier organized and spaced along the axon.
The extent to which myelination and myelin structure are dynamic and regulated by functional activity is an important question. Data are accumulating in support of mye-lination being influenced by functional activity in axons, and the molecular and cellu-lar mechanisms are being identified (Fields, 2013a). What is becoming clear is that just as synaptogenesis, synaptic plasticity, and synapse elimination are regulated by a large number of molecular and cellular processes that are influenced by functional activity, so too is activity-dependent myelination.
Functional activity can influence myelination in cell culture and animal studies, but the mechanisms are not known (Demerens et al., 1996; Makinodan et al., 2012; Liu et al., 2012; Gibson et al., 2014). Cell culture studies have identified activity-depen-dent effects on myelination by changes in cell surface molecules, diffusible signals from axons,and astrocytes responding to action potentials in axons.Neural impulse activity modulates L1- CAM expression on dorsal root ganglion (DRG) axons in cul-ture (Itoh et al.,1995) depending on the action potential firing frequency (Itoh et al., 1997) and this can influence myelination by PNS glia (Stevens et al., 1998). CNS myelination is also influenced by axonal L1-CAM (Barbin et al., 2004).
Neurotransmitters are released along axons by vesicular and non-vesicular release mechanisms (Fields and Ni, 2010). Adenosine triphosphate released from axons firing action potentials acts on Schwann cells to inhibit their proliferation and development to the myelinating stage (Stevens and Fields, 2000).
Adenosine generated by electrically active axons promotes myelination by inhibiting OPC proliferation and stimulating differentiation (Stevens et al., 2002). Initiation of myelination is regulated by vesicular release of glutamate along axons that promotes the formation of an axo-glial signaling complex necessary for initiating myelination. Activity-dependent release of glutamate from axons stimulates local synthesis of myelin basic protein in oligodendrocyte processes in contact with electrically active axons, increasing myelination of active axons (Wake et al., 2011).
Exogenous growth factors added to cell cultures (BDNF or neuregulin) increase sy-naptic connectivity between cortical neurons introduced together with OPCs to DRG neuron cultures,and tetrodotoxin (TTX) treatment to block action potential firing redu- ces the amount of myelin formed on DRG axons (Lundgaard et al.,2013). Astrocytes in cell culture release leukemia inhibitory factor (LIF) in response to ATP released from axons firing action potentials, which promotes myelination by mature oligodend-rocytes (Ishibashi et al., 2006). Recent evidence for other mechanisms of activity-dependent myelination is emerging,for example,via exosome signaling (Frühbeis et al., 2013; Pusic and Kraig, 2014) and blood oxygen tension (Yuen et al., 2014).
Astrocyte Regulation of Conduction Delays
Glia associated with axons can alter action potential conduction by means other than myelination. Action potentials in axons in the alveus of the hippocampus evoke long-lasting depolarization of oligodendrocytes that are in contact with the axon through a mechanism dependent on glutamate receptors and K + channels (Yamazaki et al., 2007). In these studies, direct depolarization of oligodendrocytes by current injection increased conduction velocity of action potentials.
Glia also influence impulse conduction through non-myelinated axons in the connec-tome.Action potential width is broadened by uncaging Ca 2+ in periaxonal astrocytes that are associated with unmyelinated axons of CA3 pyramidal neurons in the hippo-campus (Sasaki et al., 2011). This regulation of axonal signaling results from depo-larizing the axon through activation of axonal AMPA and A1 adenosine receptors by glutamate and ATP released from the periaxonal astrocyte.
The resulting action potential broadening was shown to increase synaptic transmis-sion. This mechanism of regulating action potential waveform and synaptic efficacy operates by calcium signaling in astrocytes that is not associated with synapses, but rather associated with axons far from synaptic terminals.
In myelinated axons, the perinodal astrocyte that is closely associated with the node of Ranvier (Figure 1C) has been suggested to have a role in modulating axon conduction by regulating extracellular potassium (Black and Waxman, 1988).
Whether perinodal astrocytes can also influence the membrane properties, function, and morphology of the node of Ranvier is unknown,but research on perisynaptic ast- rocytes (discussed below), provides a basis for possible astrocyte regulation of axo-nal conduction. Together, these findings highlight the importance of determining the structural connectivity of astrocytes and oligodendrocytes in white matter tracts and exploring the mechanisms of activity-dependent communication between neurons and glia beyond the synapse that influence action potential propagation through the connectome.
Myelinating Glia and Network Oscillations
Functional connectivity between ensembles of neurons is determined not only by structural connectivity, but also by dynamic changes in subthreshold membrane potential and especially by oscillations in neuronal firing.Oscillations and waves of neuronal activity link neuronal firing in phase-locked mode with other neurons locally and between distant brain regions,and such oscillatory activity during sleep is impor- tant in memory consolidation (Buzsáki, 2006; Mizuseki and Buzsáki, 2014). Precise spike times increasing myelination of active axons (Wake et al., 2011).
377
Synchronous oscillations in neuronal assemblies coordinate spike times,thus regula-ting information transmission in the cortex (Castelo-Branco et al., 1998) and encode spatial position in the hippocampus (Agarwal et al., 2014). Abnormalities in brain rhythmic activity are associated with cognitive defects and neuropsychiatric disor-ders (Buzsáki and Watson,2012;Damaraju et al.,2014). The fundamental importance of brain oscillations is suggested by the observation that as the brain scales in size during evolution,the properties of brain oscillations are preserved (Buzsáki et al., 2013). Cross-scale interactions of local,regional,and global networks are responsible for much EEG oscillatory behavior (Nunez et al., 2015), and appropriate action po-tential propagation speed is necessary to facilitate binding of remote local networks through frequency,phase,covariance, coherence,and coupled oscillation of functional activity (Pajevic et al., 2014). This coordinated firing also gives rise to patterns in the electro-encephalogram (EEG),which is an important technique for studying the functional connectome.
Synchronous activity in local neuronal assemblies generates fluctuation in local field potentials (oscillations), which can be recorded on the surface of the scalp via EEG. Astrocytes influence neuronal membrane potential and excitatory and inhibitory synaptic transmission underlying neural oscillations locally (discussed below),and as discussed,oligodendrocytes provide rapid impulse propagation to sustain long-range oscillations and synchrony of spike time arrival between distant populations of neurons. Thus, interactions within populations of neurons locally and across distant regions of the brain are essential in all but the most rudimentary forms of information processing and learning.Thus,glial cells contribute in several ways to coupling activi- ty in ensembles of neurons in local circuits and across long-distance networks in the brain.Moreover,this broad spatial integration across distant brain regions is achieved across exceedingly wide temporal scales ranging well beyond the millisecond to seconds of electrical signaling typically recorded in neurons, to encompass instead hours, days, and months. These longer time frames are well-suited to the temporal dynamics of glial communication and plasticity.
Local Network Connectivity
Determining the structural and functional connectivity in local networks is an impor-tant goal of connectomics, but both of these properties of local circuits are influenced by glia in several ways.
Understanding of local networks will be inadequate if the structure and function of glia in local networks is ignored.
Neuron-Glia Interactions in Local Circuit Function
Astrocytes are integrated into local synaptic functioning in the adult brain. Cytoplas-mic Ca 2+ transients in astrocytes in adult cerebral cortex are generated in response to whisker stimulation.
The astrocyte response to sensory stimulation is partly mediated by synaptic release of glutamate acting on astrocyte mGluR5 receptors (Wang et al., 2006). Activation of intracellular Ca 2+ signaling in astrocytes can augment or suppress both excitatory and inhibitory synaptic transmission and influence state-dependent changes in corti-cal activity, for example, sleep (Halassa et al., 2009) and working memory. Calcium signaling in astrocytes in response to glutamate and GABA neurotransmission functionally segregates large volumes of neuropil in the mossy fiber pathway of the hippocampus (Haustein et al.,2014).Astrocytes,acting through a receptor-mediated pathway, can also modulate extracellular K + and regulate neuronal spiking activity (Wang et al., 2012). Purine signaling from a single astrocyte produces an UP state (persistent depolarization resulting in high-frequency firing) in a population of neigh-boring neurons (Poskanzer and Yuste, 2011). Astrocytes working in conjunction with synaptic plasticity create neuronal clusters that become associated with particular in-put signals. There is a need to determine both the anatomical organization of astro-cytes in gray and white matter and identify how they interact with neurons, synapses and nodes of Ranvier to modulate information transmission through neural networks.
The importance of waves and oscillations of neural activity in connecting distant re-gions of the brain have been mentioned, but oscillations in local circuits in gray mat-ter are also important for information processing.These oscillations are driven prima- rily by inhibitory neurons and they are known to be modulated by sensory stimuli and behavioral states.Oscillations in the gamma frequency band (25-80 Hz),for example, are correlated with learning,memory storage and retrieval, attention,and other cogni- tive or motor functions (Bas x ar-Eroglu et al., 1996). Neurotransmitter release from astrocytes via vesicle fusion has recently been shown to be necessary to maintain fast (25Hz) dynamics of local neural circuits and to sustain normal cognitive behavior (Lee et al.,2014).This was revealed by blocking transmitter release by expressing tetanus neurotoxin delivered through a lentiviral vector into astrocytes and also by developing a transgenic mouse enabling reversible induction of tetanus toxin expres-sion in astrocytes. This manipulation of astrocytes reduced gamma oscillations that are induced by carbachol in hippocampal slices. In these animals, the gamma frequency of EEG power was decreased in awake behaving mice.
Moreover, performance in novel object recognition was impaired. Other forms of me-mory (Y-maze and fear conditioning) were preserved.Previously fast cortical oscilla-tions in neural activity were thought to be produced entirely by neurons. These new findings show that gamma oscillations are specifically involved in recognition memo-ry, and astrocyte transmission dependent on exocytosis has a crucial influence on fast information processing and cognitive behavior. Thus, the same logic that makes
tracing the connectome of interneurons necessary also applies to astrocytes in gray matter.
Heterogeneity of Astrocytes in Local Neural Circuits
Evidence suggests that astrocytes have microcircuit-specific properties. Astrocytes release multiple neurotransmitters, including glutamate (Jourdain et al.,2007; Woo et al.,2012),ATP (Lalo et al.,2014),D-serine (Henneberger et al., 2010), and GABA (Lee et al.,2010),that can have multiple complex influences on synaptic transmission. Astrocytes also regulate clearance of neurotransmitter from the synaptic cleft (Djukic et al., 2007), further influencing synaptic transmission and functional coupling of synapses (Perea and Araque, 2007).
378

Figure 2. Astrocytes Are Intimately Associated with Tens of Thousands of Synapses through Highly Ramified Slender Branches Astrocytes can influence neuronal con-nectivity by binding multiple synapses and multiple neurons into functional assemb-lies, but astrocytes also operate at a subcellular level to sense and modulate synaptic activity at single synapses.
(A) A single astrocyte from the neocortex of an adult mouse; note the cell body, multiple branches, and intricate fine highly branched terminals.
(B) An enlargement and surface rendering of the astrocyte processes shown in (A). Courtesy of Eric Bushong and Mark Ellisman at the National Center for Microscopy and Imaging Research (University of California, San Diego). See Shigetomi et al. (2013) for additional information. The large tick marks are 5 mm in (A) and 0.5 mm in (B).
Different types of neurotransmitter receptors responding to the same ligand further add to the complexity of astrocyte regulation of synaptic transmission. For example, ATP and adenosine receptors on neurons often produce opposite cellular responses in a manner that can be homeostatic (Fields and Burnstock,2006).Extracellular enzy- matic activity generating adenosine from ATP released from astrocytes thus affects the dynamics of astrocyte regulation of synaptic function in complex ways.To cite an-other example, A1 adenosine receptors on CA1 neurons depress synaptic transmis-sion (Pascual et al.,2005; Serrano et al.,2006), but glutamate, and glutamate release from astrocytes, potentiates synaptic transmission through mGluR-dependent presy-naptic mechanisms (Navarrete and Araque,2010; Fiacco and McCarthy,2004). Much of the apparent ‘‘controversy’’ that has been ascribed to astrocyte signaling and in-teractions with neurons is likely the result of oversimplification and over-extrapolation from an extremely limited knowledge base.This vast and complex information about neuron-glial interactions must be obtained to fully understand how neural networks operate.
An additional level of complexity is that astrocytes have different properties and functions in different brain regions.
Even in different subfields of the hippocampus calcium responses in astrocytes and the interactions with neurons differ (Haustein et al.,2014). In the hippocampal mossy fiber pathway less active calcium signaling is observed in finely ramified processes of astrocytes than in other hippocampal regions. These responses are not due to mGluRs,NMDA receptors (NMDARs),or action potential firing as they can be in other brain regions. Astrocytes in this subregion of hippocampus require prolonged bursts of action potentials to elicit astrocytic calcium responses that have comparatively slow dynamics rather than single action potential sensitivity, as in the stratum radia-tum of CA1, that generates rapid calcium responses in discrete subcellular astrocytic domains. In some subfields of hippocampus, astrocytes also respond to neuronal transmission by activation of GABAb receptors, but in other regions they are activated by nonselective cation channels such as TRPA1 (Shigetomi et al., 2013), mGuR2/3 (Haustein et al., 2014), or mGluR5 (Fiacco and McCarthy, 2004; Perea and Araque, 2007) receptor or purinergic receptor activation (Pascual et al., 2005). Astrocyte signaling, as well as synaptic transmission between neurons, is regulated by glutamate clearance. When glutamate uptake is blocked, astrocytes can exhibit enhanced spontaneous Ca 2+ signals (Haustein et al., 2014). This response in the mossy fiber pathway contrasts with astrocyte Ca 2+ signaling and modulation of synaptic transmission in other hippocampal subfields, as will be discussed below.
Astrocytes in Local Network Structure
Astrocytes have anatomical and physiological properties that can impose a higher order organization on information processing and integration in the neuronal connec- tome (Figure 2). The volume of human astrocytes is almost 20-fold larger than their rodent counterparts (Oberheim et al.,2006),enabling a human cortical astrocyte to integrate input from #2 million synapses compared with 0.1 million in the rodent brain. In the gray matter regions of cerebral cortex and hippocampus, astrocytes are organized in non-overlapping domains (Bushong et al., 2002).
The significance of this tile-like organization is not known but has the potential to couple local neuronal circuits into functional assemblies and provide homeostatic regulation of large populations of neurons to prevent hyper- or hypo-excitability or provide regional control over information processing. The degrees of freedom for in- formation storage are multiplied beyond synapse-specific connectivity by astrocytes modulating synaptic strength and coupling domains of synapses into functional as-semblies. Evidence in support of this idea comes from experiments on mice in which a large proportion of astrocytes were replaced with human astrocytes. These mice exhibited increased synaptic plasticity and faster learning (Han et al.,2013).This sup-ports the hypothesis that astrocytes participate in synaptic plasticity and learning and that the unusual features of human astrocytes contribute to the superior cognitive abilities of the human brain.
379
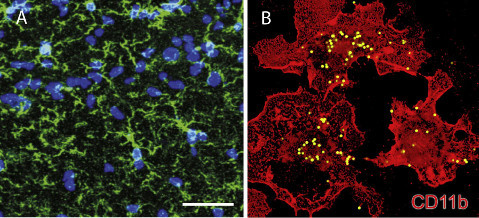
Figure 3. Microglia Respond to Nervous System Damage but Also Monitor and Remove Synapses in an Activity-Dependent Manner
(A) Microglia in a resting state in the CA1 area of hippocampus (green, Iba-1), nuclei are blue.
(B) Activated microglia in vitro engulfing fluorescent-labeled latex beads. (A) From Zhang et al. (2014). (B) From Black and Waxman (2013). Neuron Perspective
Astrocytes also promote synapse formation, influence synapse stability, and help refine neural connectivity.Astrocytes participate during development in the formation of synaptic networks by regulating synaptogenesis (Molofsky et al.,2012; Allen et al., 2012; Eroglu et al., 2009) and by pruning synaptic spines (Chung et al., 2013). Very little information is available on the important question of how these activities are carried out in a region-restricted manner by astrocyte heterogeneity.It is known that astrocytes express several region-specific genes. For example, ventral spinal cord astrocytes encoding semaphorin3 a (Sema3a) are required for proper motor neuron and sensory neuron circuit organization (Molofsky et al., 2014). Loss of astrocyte-encoded Sema3A leads to dysregulation of alpha-motor neuron axon initial segment orientation, abnormal synaptic inputs, and selective death of these neurons, as well as ectopic projection of sensory afferents into ventral positions. This demonstrates that astrocytes influence sensory-motor circuit formation and that regional astrocyte heterogeneity helps coordinate postnatal neural circuit connectivity and refinement. Such findings highlight the need to understand glial structure and function to comprehend how the neural connectome forms, operates, is modulated adaptively, becomes dysfunctional in disease and recovers after injury.
Neuronal structural plasticity and spine dynamics are also regulated by astrocytes. Synapses are enveloped with intricate slender processes of astrocytes that are diffi-cult to visualize without electron microscopy (Figure 2B); these are highly motile structures exhibiting rapid actin-dependent movements accompanying Ca 2+ ele- vations. Moreover, in hippocampus synaptic activity acting through astrocytic meta- botropic glutamate receptors and intracellular calcium signaling promotes motility of these perisynaptic astrocyte processes (Bernardinelli et al., 2014).
Synaptic activation that induces long-term potentiation causes transient increased mobility of the fine astrocytic processes.This increases astrocytic coverage of the synapse and stabilizes the spine, which promotes memory consolidation. In contrast to activity-dependent neuronal plasticity, little is known of astrocyte plasticity in response to neuronal firing (Xie et al., 2014).
In addition to astrocytes, microglia also participate in activity- dependent pruning of synapses (Paolicelli et al.,2011; Schafer et al.,2012) (Figure 3). Microglia also trigger long-term synaptic depression (LTD) through activation of the complement receptor 3 on microglia when hypoxia and inflammation are combined (Zhang et al., 2014). Considering the motility of glia in probing dendrites and the types of neuromodulato-ry factors these cells can secrete, microglial involvement in synaptogenesis seems possible and worthy of further investigation.
Astrocyte Coupling of Neuronal Functional Assemblies by Metabolic and Vascular Coupling Astrocytes associated with blood vessels shuttle ions and energy subst-rates between neurons and the blood stream. The metabolic support astrocytes pro-vide to neurons is critical for synaptic function. The large area of astrocyte coverage in the neuropil and the extensive interconnection among astrocytes through gap junctions can influence local neural circuit function on relatively large scales. Studies using a fluorescent analog of glucose to track glucose and lactate diffusion through transcellular networks of astrocytes in hippocampus, show that increased energetic demand by neurons generates a diffusion gradient for glucose within astrocytic net-works. This draws glucose through distant cells in the astrocyte network and ultima-tely from perivascular astrocytes. Glucose provided by astrocytic networks sustains synaptic transmission during glucose depletion and during epileptic activity (Rouach et al., 2008).
This demonstrates the importance of astrocytes in a network communicating through connexins to support neuronal activity during stress and disease,but it also suggests the possibility that similar regional distribution of glucose to subsets of neurons could operate during normal physiological function to coordinate domains of neuronal func-tion. Transient disruption of the flow of energy substrates from astrocytes to neurons has been shown to severely impair formation of long-term memory (Suzuki et al., 2011).
There is no question that astrocytes are involved in actively coupling neuronal activi-ty in local populations through regulation of local blood flow (Moore and Cao, 2008; Lind et al., 2013).
Indeed, one of the primary methods to study the functional neuronal connectome is via blood oxygenation level-dependent (BOLD) fMRI, a technique based on the regulation of blood oxygen in local populations of neurons that are actively firing.
Perivascular astrocytes control local blood flow, and whether astrocytes induce local vasodilation or vasoconstriction in response to neural activity depends on the oxy-gen levels in brain tissue.When oxygen availability is low astrocytes release lactate, which increases prostaglandin E2 levels, stimulating vasodilation (Gordon et al., 2008). Under normal oxygen conditions, arterioles constrict as a result of astrocyte-derived arachidonic acid following increased neural activity or increased intracellular calcium concentration in astrocytes.
380
Thus fMRI signals may be viewed as indirect measures of astrocyte activity as well as neuronal activity,although how to distinguish between them is not clear. Without a better understanding of astrocyte structure and functional, the utility of fMRI in dedu-cing functional activity in the neural networks may be limited.Moreover,such dynamic regional control of neural activity is occurring constantly in the brain as an essential part of normal information processing in neural networks.
Presumably if this astrocyte-regulation of local blood flow is deficient, perturbations of neuronal functional connectivity would be expected.
Subcellular Connectivity
The neural connectome at the most elemental level - individual synapses - is coupled to astrocytes in a cellular network that is only superficially understood.
Although astrocytes are large and morphologically complex cells, they also operate with exquisite subcellular segregation in discrete cellular compartments, enabling as-trocytes to interact with individual synapses. In addition to cell-wide changes in cyto-plasmic calcium, rapid, spontaneous Ca 2+ transients are seen in subcellular com-partments of astrocytes by using 2-photon imaging with genetically encoded calcium indicators (Haustein et al.,2014).Moreover,astrocytes can sense and regulate activity at single synapses. Astrocytic signaling participates in some forms of synaptic plasti-city that are synapse-specific; i.e., cholinergic-induced LTP in cortex and hippocam-pus (Navarrete et al.,2012); spike-timing-dependent cortical plasticity, hippocampal LTD and working memory (Han et al.,2012), and LTP has been shown to depend on D-serine release from astrocytes (Henneberger et al., 2010).
The complex branched cellular processes of astrocytes can show intense local Ca 2+ activity triggered by physiological transmission in nearby synapses (Di Castro et al., 2011). These slender astrocyte processes can also sense spontaneous synaptic events, which cause local changes in Ca 2+ in subcellular domains of astrocyte pro-cesses.Astrocytes have been shown to detect synaptic activity induced by single ac- tion potentials, and in response, they upregulate basal synaptic transmission (Pana-tier et al., 2011). Thus, in the CA1 region of hippocampal slice preparations, astro-cytes are as sensitive as neurons in detecting synaptic activity. Blocking calcium signaling in these astrocytes with BAPTA greatly decreases basal synaptic strength through a presynaptic mechanism dependent on A2A presynaptic receptors.
Activation of mGluR5 receptors is necessary for these astrocytes to sense synaptic transmission and to evoke an astrocytic calcium response, but the release of ATP and rapid hydrolysis to adenosine is responsible for increasing synaptic transmission by acting on presynaptic terminals (Panatier et al., 2011).
These examples highlight the importance of astrocytes in synaptic functional con-nectivity and synaptic plasticity, but the mechanisms of astrocyte regulation of indivi-dual synaptic function are only beginning to be identified. As mentioned, microglia also modify subcellular connectivity by selectively pruning individual synapses in an activity-dependent manner (Schafer et al., 2012; Paolicelli et al., 2011; Tremblay et al., 2010).
Summary and Future Directions
Connectivity and communication among the major types of glia are only beginning to be explored. The neuronal connectome was established by anatomical methods, no-tably histology, neuronal tracing,live-cell confocal microscopy, electrophysiology, and human brain imaging. A major goal of the BRAIN Initiative
(http://www.braininitiative.nih.gov/index.htm)
is to develop new methods to study neural connectivity (Devor et al., 2013). These tools will be useful for understanding glial function and connectivity,but new methods exploiting the different types of glial connectivity, function, and signaling will be requi-red to understand how different types of glia are organized and interact with each other and with neurons in different brain regions. Determining structural and functio-nal connectivity of glia and their interactions with neurons will be essential to under-standing the neuronal connectome (Fields, 2013b). Oligodendrocyte progenitor cells (OPCs), however,are a part of the neuronal connectome even by strict definition, be- cause axons form synapses onto these glial cells (Bergles et al., 2000), for reasons that are unknown.
Astrocyte heterogeneity in gray matter of different brain regions is only beginning to be explored. Less is known of astrocytes in white matter (Butt et al.,1994), and white matter comprises half of the human brain. It is unclear how many kinds of astrocytes there are, what their anatomical distribution is within the brain and spinal cord, and how astrocyte structural and functional heterogeneity integrates with neuronal diversity and function. Similar questions pertain to microglia and oligodendrocytes.
How plastic glial properties are in association with brain function and dysfunction needs to be determined.Oligodendrocyte structure,changes in oligodendrocytes, and interactions between myelinating glia and other glia (astrocytes and microglia) are not well understood. White matter structure can change with functional activity and environmental experience, but the cellular basis for changes in white matter seen by human brain imaging after learning are not clearly identified (Zatorre et al., 2012).
In comparison to the detailed understanding of neuronal communication, how glia communicate with other glial cells and with neurons is only beginning to be explored. The known mechanisms include diffusible signaling molecules, gap junctions, and membrane potential changes, but determining what signaling molecules, receptors, and release mechanism are involved will require years of research. How these me-chanisms of communication and functional interactions with neurons change during development, in different physiological states, in pathology, and in response to neuronal activity are largely unknown.
There are major current questions concerning the contribution of glia in nervous sys-tem plasticity. Among these are how plastic is myelin and the node of Ranvier in the normal brain and does this plasticity promote optimal network function and learning? How do glia participate in generating extracellular field potentials and promote oscillations and synchrony of neuronal firing?
381
Do changes in astrocytes map with changes in synaptic strength or neuronal firing during memory encoding and in association with other forms of information proces-sing in neural networks? What is the function and fate of progenitor oligodendrocytes (NG2 glia), which populate the entire brain uniformly and represent the largest popu-lation of dividing cells in the adult brain? An important area for future research will be to determine how different types of glia regulate synaptic function and plasticity, neu-ronal excitability, and impulse propagation, as well as regulate various types of glia. Neurons compute via membrane voltage, but how do astrocytes compute? What do glia contribute to information processing that neurons cannot accomplish and how will incorporating glial biology help address long-standing problems in neuroscience? Exploring glial interaction with the neuronal connectome will require development of new tools and most likely expanding concepts on the cellular basis of brain function.
Tools for Studying Glial Regulation of the Neuronal Connectome
Calcium imaging is what revealed glial communication and interactions with neurons, but this method has been available for 25 years, and glia use many other mechanisms of communication.
New techniques are needed to monitor astrocyte (and other glial cell) signaling. New optogenetic methods are needed to selectively activate different kinds of glia. Using light-gated ion channels to activate glia does not,in general, replicate the normal me- chanism of glial communication because these cells do not generate action poten-tials or operate as neurons do primarily by changes in membrane potential. Glia uti-lize ligand-gated channels,membrane receptors,release of neurotransmitters, growth factors,and neuromodulators for intercellular communication,but the available means of manipulating these signaling pathways with the proper spatial and temporal properties mimicking normal physiological conditions are inadequate. For example, just as with neurons, where treatment with glutamate is a weak approach to study synaptic plasticity, the same difficulty pertains to glial biology.
Photostimulation of channelrhodopsin-2 expressed in astrocytes causes glutamate release that activates AMPA receptors on Purkinje cells to induce long-term depres-sion of synapses through activation of mGluRs (Sasaki et al.,2012).However,there is uncertainty as to how physiological this stimulation via channelrhodopsin is for glial cells, considering that membrane depolarization is not how these cells are generally activated in vivo.
One interpretation using this method is that intracellular acidification resulting from proton influx through ChR2 could mimic the acidosis that occurs during ischemic brain damage. This acidification is accompanied by glutamate release (Beppu et al., 2014).
Achieving specific stimulation of targeted glia can be difficult because the membrane receptors and signaling molecules involved are often shared by many types of glia and with neurons. Genetic expression of exogenous receptors in glial cells is a help-ful approach to obtaining stimulus specificity, but stimulating the receptors with the proper temporal dynamics and appropriate subcellular distribution presents difficul-ties. The consequences of glial signaling are most often analyzed by the resulting effect on neurons, but this is an indirect measure of glial function.
Microglial biology is difficult to study under normal conditions because these cells are activated in response to injury that is required to expose the brain for study. New standardized experimental models and preparations for studying glia and glial plasti-city are needed. Mathematical modeling should be developed for glial functions and their interactions with neurons.
Development of human brain imaging methods to ascertain glial structure and chan-ges, including myelination, migration of microglia, astrocyte communication, altera-tions,and interactions with neurons and blood vessels,is an important and necessary direction for future research.
Recent data from human brain imaging by MRI, notably using diffusion tensor ima-ging (Basser et al., 1994), has revealed structural changes in myelinated tracts after learning a wide range of tasks (Zatorre et al.,2012). The cellular basis of these chan- ges in white matter is being investigated, but present evidence supports some contri-bution by glia (astrocytes and myelin). However, the current state of the art is that even the most rudimentary features of myelin structure in neural networks are far from being understood even in experimental animal models where microscopic ana-lysis of tissue is possible. Partly this is a result of the neurocentric view of neural net-work function that has relegated the field of myelin research largely to pathology and developmental neuroscience, but primarily it is because of technical limitations.
The principal challenge in determining oligodendrocyte contribution to the brain connectome is that the task requires spanning an enormous range of spatial scales. Myelin structure is submicroscopic, whereas neural large-scale networks are macro-scopic. Methods of automating the collection of serial section EM data are permitting reconstruction of large volumes of neural networks with subcellular resolution(Figure 1A) (Lichtman and Denk,2011;Bock et al.,2011; Kleinfeld et al.,2011). Where this has been accomplished, surprising new anatomical features of myelin at the circuit level are being observed that have profound effects on network connectivity and function.
For example, pyramidal neurons of cortical layers II/III, IV, and V show different pro-files of myelination (Tomassy et al.,2014). In layer II/III, individual axons of pyramidal neurons have long stretches (up to 55 mm) of unmyelinated segments interspersed with myelinated segments. Variation in myelination along axons could adjust trans-mission speed for optimal synchrony, or permit more complex forms of network inte-gration. Synapses can form on unmyelinated segments of axons (Somogyi et al., 1998) and bare axons can release neurotransmitters (Fields and Ni, 2010) that can stimulate glia or other neurons.
Action potential propagation along the axon and back propagation of the action po-tential into the cell body and dendrites are influenced by myelination and notably by the axon initial segment (AIS),the 5–80 mm-long unmyelinated segment between the cell body and first myelin segment where the action potential is triggered. Back pro-pagation of action potentials can affect neural integration and synaptic plasticity (Bu-kalo et al.,2013). The morphology of the AIS and the types of ion channels present in it regulate excitability of the neuron, influence the shape of the action potential, the frequency of action potential firing and other aspects of action potential signaling (Palmer and Stuart, 2006).
442
The AIS is longer in layers III/IV than in V/VI,indicating that layer-specific myelination of axons can influence action potential propagation in cortical networks. Thus myelin can constrain where axons sprout and form synapses with dendrites and other axons. Axon growth inhibitory proteins in the myelin sheath, such as Nogo (Schmandke et al., 2014), block axon sprouting, stabilizing the structure and pattern of connectivity of neural circuits.
Genetic techniques, notably to identify different populations of glia, and also genetic manipulation will be helpful in future research on glial connectivity and function. In Drosophila,for example,genetic ablation of subsets of astrocytes results in expansion of territory of the remaining cells,indicating self-repulsive interactions in limiting terri-torial size (Stork et al.,2014) as a likely basis for the non-overlapping domains of ast- rocytes observed in mammalian cortex (Bushong et al.,2002).This genetic control of astrocyte networks in combination with monitoring effects on neural network function will be a powerful approach to understand how astrocytes may couple large domains of synapses or neurons into larger functional assemblies.
Oligodendrocyte regulation of axonal conduction velocity through myelination has a profound effect on connectome function. New methods to study myelin structure are desirable and they are being developed. Myelin, being highly non-polar, will provide a barrier to water diffusion and affect the mobility of water protons in tissues. Thus, new methods are being developed to probe myelin microstructural changes in the human brain non-invasively by diffusion MRI. The sub-cellular resolution required to infer myelin structure is still finer than the diffusion distances these methods can currently probe. Given the unique structure of myelin, with its multilaminar layers of lipid membranes,more selective probes for myelin structure would seem possible, for example by using myelin-selective contrast agents.
Other MR relaxation mechanisms could be measured in conjunction with diffusion MRI to assess microstructural and compositional characteristics of myelin. MRI methods that can selectively interrogate different populations of water molecules within and around myelin, and assessing their exchange should also help inform our understanding of myelin structure and function relationships.
The importance of conduction delays through the connectome has been empha-sized, raising the question of whether or not it is possible to measure conduction delays or latencies along white matter pathways non-invasively and in vivo. Recent progress has been made on this front by combining various in vivo MRI methods with classical neurophysiology (Avram and Basser,2014). Specifically, Brodmann-like areas are first identified using established MRI-based cortical parcellation methods (Fischl et al.,1999a, 1999b,2009). Diffusion MRI ‘‘Tractography’’ is then used to iden- tify white matter pathways connecting them,from which their mean pathlength can be determined (Basser et al., 2000; Wedeen et al., 2008). Axon diameter or diameter distributions measurements are made along these pathways (Assaf et al., 2008; O ̈zarslan et al., 2013). From these data, mean conduction times can then be inferred from these diameter data using classical neurophysiological relationships between conduction velocity and axon diameter (Hursh, 1939; Tasaki et al., 1943; Waxman and Bennett,1972). In summary, by knowing the path length of fascicles and inferring their conduction veloc-ity, one can now estimate the mean transit times or latencies between different brain areas or regions (Avram and Basser, 2014).
While preliminary, this approach holds out the promise of in vivo whole-brain latency mapping as a means to assess gross anatomical network function; possibly explai-ning normal and abnormal developmental delays, changes, or deficits observed in disease or injury (e.g., mild TBI), and even enhancements due to learning or training (Gärtner et al., 2013).
While it is simplistic to believe that axon diameter alone is the only determinant of conduction velocity (Waxman, 1980), as it is affected by myelin quality and quantity, nodal properties, and myelin thickness, axonal membrane properties, among others; progress is being made to measure and quantify each of these determinants of con-duction velocity. For instance,several MR methods exploiting differences in magnetic susceptibility in different axonal components have been proposed to measure myelin and myelin-water content. Others have been proposed to measure the well-known g-ratio (Stikov et al.,2011) that characterizes myelin thickness. In general, prospects appear bright for future developments of new functional imaging methods that integrate microstructural MRI data.
Concluding Remarks
In addition to new techniques, conceptual advances will be required to understand how glia operate as a system and interact with neural networks and subcellular domains of neurons. In comparison to neurons, glia communicate slowly and over broader spatial scales. This may make glia particularly well- suited for involvement in integration, in homeostatic regulation, and alterations in structural or functional connectivity of neural networks taking place over periods of weeks or months.
Involvement of all three major types of CNS glia in wide-ranging disorders through glial effects on the neuronal connectome are evident; for example, TREM2 is highly expressed by microglia and mutations in this gene are a risk factor for Alzheimer’s disease (Kleinberger et al., 2014). Astrocytes have been linked to rapidly acting ef-fects of sleep deprivation on depression. Antidepressant effects of sleep deprivation require astrocyte- dependent adenosine-mediated signaling (Hines et al., 2013).
Astrocytes, microglia, and oligodendrocytes are implicated in amyotrophic lateral sclerosis (ALS) (Leblond et al., 2014). These are a few examples of how pervasive glial influences are on health through interactions with the neuronal connectome, and this provides a pervasive argument that a sound foundation for understanding the basic biology of glia is critical to basic science and human health.
In contrast to neurons, where the mechanism of cellular computation and signaling is believed to be based on electrical membrane potential, computation in astrocytes and other glia is not understood. The emphasis on calcium signaling is likely a result of the favorable development of fluorescent imaging to study calcium signaling rather than Ca 2+ being the only mechanism of glial signaling and integration. Com-putation in glial cells appears to be more consistent with the complex biochemical networks in cell biology, which are much less studied in neuroscience.
383
The argument is sometimes made that glial structure and function will be revealed as a byproduct of studying the neuronal connectome. But the brain, like all organs, is a multicellular structure and its operation cannot be understood by analysis of only one cell type in it or by viewing the function of all brain cells exclusively through the lens of neuronal function.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program, The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NIH.
REFERENCES
Agarwal, G., Stevenson, I.H., Berényi, A., Mizuseki, K., Buzsáki, G., and Sommer, F.T. (2014). Spatially distributed local fields in the hippocampus encode rat position. Science 344, 626–630.
Allen, N.J., Bennett, M.L., Foo, L.C., Wang, G.X., Chakraborty, C., Smith, S.J., and Barres, B.A. (2012). Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 486, 410–414.
Assaf, Y., Blumenfeld-Katzir, T., Yovel, Y., and Basser, P.J. (2008). AxCaliber: a method for measuring axon diameter distribution from diffusion MRI. Magn. Reson. Med. 59, 1347–1354.
Bushong, E.A., Martone, M.E., Jones, Y.Z., and Ellisman,M.H. (2002). Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 22, 183–192.
Butt, A.M., Duncan, A., and Berry, M. (1994). Astrocyte associations with nodes of Ranvier: ultrastructural analysis of HRP-filled astrocytes in the mouse optic nerve. J. Neurocytol. 23, 486–499.
Buzsáki, G. (2006). Rhythms of the Brain. (Oxford, UK: Oxford University Press).
Buzsáki, G., and Watson, B.O. (2012). Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin. Neurosci. 14, 345–367.
Buzsáki, G., Logothetis, N., and Singer, W. (2013). Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron 80, 751–764.
Castelo-Branco,M., Neuenschwander,S., and Singer,W. (1998). Synchronization of visual responses between the cortex, lateral geniculate nucleus, and retina in the anesthetized cat. J. Neurosci. 18, 6395–6410.
Chung, W.S., Clarke, L.E., Wang, G.X., Stafford, B.K., Sher, A., Chakraborty, C., Joung, J., Foo, L.C., Thompson, A., Chen, C., et al. (2013). Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400.
Avram, A.V., and Basser, P.J. (2014). Inferring millisecond-scale functional connec-tivity from tissue microstructure. In Joint Annual Meeting ISMRM-ESM-RAB (Italy: Milan), May 10-16,Abstract No. 6968.
Cooper, N.G. and Steindler, D.A. (1986). Monoclonal antibody to glial fibrillary acidic protein reveals a parcellation of individual barrels in the early postnatal mouse somatosensory cortex. Brain Res. 380, 341–348.
Barbin, G., Aigrot, M.S., Charles, P., Foucher, A., Grumet, M., Schachner, M., Zalc, B., and Lubetzki, C. (2004). Axonal cell-adhesion molecule L1 in CNS myelination. Neuron Glia Biol. 1, 65–72. Damaraju, E., Allen,E.A., Belger,A., Ford,J.M., McEwen, S., Mathalon, D.H., Mueller, B.A., Pearlson,G.D., Potkin,S.G., Preda,A.,et al. (2014). Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clin. 5, 298–308.
Barkho, B.Z., Song, H., Aimone,J.B., Smrt,R.D., Kuwabara,T., Nakashima, K., Gage, F.H., and Zhao, X. (2006). Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 15, 407 – 421.
Bas har-Eroglu,C., Struber,D., Schu ̈rmann, M.,Stadler, M., and Bas har, E. (1996). Gamma-band responses in the brain: a short review of psychophysiological correlates and functional significance. Int. J. Psychophysiol. 24, 101–112.
Basser, P.J., Mattiello, J., and LeBihan, D. (1994). MR diffusion tensor spectroscopy and imaging. Biophys. J. 66, 259–267.
Basser, P.J., Pajevic, S., Pierpaoli, C., Duda, J., and Aldroubi, A. (2000). In vivo fiber tractography using DT-MRI data. Magn. Reson. Med. 44, 625–632.
Beppu, K., Sasaki, T., Tanaka, K.F., Yamanaka, A., Fukazawa,Y., Shigemoto, R., and Matsui, K. (2014).Optogenetic countering of glial acidosis suppresses glial glutamate release and ischemic brain damage. Neuron 81, 314–320.
Bergles, D.E., Roberts,J.D., Somogyi,P., and Jahr,C.E. (2000).Glutamatergic synap- ses on oligodendrocyte precursor cells in the hippocampus. Nature 405, 187 – 191.
Bernardinelli, Y., Randall, J., Janett, E., Nikonenko, I., König,S., Jones, E.V., Flores, C.E., Murai,K.K., Bochet,C.G., Holtmaat,A., and Muller,D. (2014).Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr. Biol. 24, 1679–1688.
Black, J.A., and Waxman, S.G. (1988). The perinodal astrocyte. Glia 1, 169 – 183.
Black, J.A., and Waxman, S.G. (2013). Noncanonical roles of voltage-gated sodium channels. Neuron 80, 280–291.
Bock, D.D., Lee, W.C., Kerlin, A.M., Andermann, M.L., Hood, G., Wetzel, A.W., Yurgenson, S., Soucy, E.R., Kim, H.S., and Reid, R.C. (2011). Network anatomy and in vivo physiology of visual cortical neurons. Nature 471, 177–182.
Brown, A.M., Tekko ̈k, S.B., and Ransom, B.R. (2003). Glycogen regulation and functional role in mouse white matter. J. Physiol. 549, 501–512.
Bukalo, O., Campanac,E., Hoffman,D.A., and Fields,R.D. (2013). Synaptic plasticity by antidromic firing during hippocampal network oscillations. Proc. Natl. Acad. Sci. USA 110, 5175–5180.
Dean, D.C., 3rd, O’Muircheartaigh, J., Dirks, H., Waskiewicz, N., Walker, L., Doern-berg, E., Piryatinsky, I., and Deoni, S.C. (2014). Characterizing longitudinal white matter development during early childhood. Brain Struct. Funct.
http://dx.doi.org/10.1007/s00429-014-0763-3.
Demerens, C., Stankoff, B., Logak, M., Anglade, P.,Allinquant, B., Couraud, F., Zalc, B., and Lubetzki, C. (1996). Induction of myelination in the central nervous system by electrical activity. Proc. Natl. Acad. Sci. USA 93, 9887–9892.
Devor, A., Bandettini, P.A., Boas, D.A., Bower,J.M., Buxton,R.B., Cohen, L.B., Dale, A.M., Einevoll, G.T., Fox, P.T., Franceschini, M.A., et al. (2013). The challenge of connecting the dots in the B.R.A.I.N. Neuron 80, 270–274.
Di Castro, M.A., Chuquet, J., Liaudet, N., Bhaukaurally, K., Santello, M., Bouvier, D., Tiret, P., and Volterra, A. (2011). Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 14, 1276–1284.
Diniz, L.P., Almeida, J.C., Tortelli, V., Vargas Lopes, C., Setti-Perdiga ̃o, P., Stipursky, J., Kahn, S.A., Roma ̃o, L.F., de Miranda, J., Alves-Leon, S.V., et al. (2012). Astro-cyte-induced synaptogenesis is mediated by transforming growth factor b signaling through modulation of D-serine levels in cerebral cortex neurons. J. Biol. Chem. 287, 41432–41445.
Djukic, B., Casper, K.B., Philpot, B.D., Chin, L.S., and McCarthy, K.D. (2007). Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J. Neurosci. 27, 11354–11365.
Eroglu, C., Allen, N.J., Susman, M.W., O’Rourke, N.A., Park, C.Y., Ozkan, E., Chak-raborty, C., Mulinyawe, S.B., Annis, D.S., Huberman, A.D., et al.(2009). Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 139, 380-392.
Fiacco, T.A., and McCarthy, K.D. (2004). Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. J.Neurosci. 24, 722-732.
384
Fields, R.D. (2005). Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist 11, 528–531.
Fields, R.D. (2008). White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 31, 361–370. Neuron 86, April 22, 2015 a2015 Elsevier Inc.
Fields, R.D. (2013a). Regulation of myelination by functional activity. In Neuroglia, Third Edition, H. Kettenmann and B.R. Ransom, eds. (Oxford,UK: Oxford University Press). Hines, D.J., Schmitt, L.I., Hines, R.M., Moss, S.J., and Haydon, P.G. (2013). Antidepressant effects of sleep deprivation require astrocyte-dependent adenosine mediated signaling. Transl. Psychiatr. 3, e212.
Fields, R.D. (2013b). Neuroscience: map the other brain. Nature 501,25–27. Hirrlin-ger, J., and Nave, K.A. (2014). Adapting brain metabolism to myelination and long-range signal transduction. Glia 62, 1749–1761.
Fields, R.D. (2014). Neuroscience. Myelin — more than insulation. Science 344, 264 – 266.
Fields, R.D.,and Burnstock,G.(2006).Purinergic signalling in neuron-glia interactions. Nat. Rev. Neurosci. 7, 423–436.
Fields, R.D., and Ni, Y. (2010). Nonsynaptic communication through ATP release from volume-activated anion channels in axons. Sci. Signal. 3, ra73.
Fischl, B., Sereno, M.I., and Dale, A.M. (1999a). Cortical surface-based analysis. II: Inflation,flattening,and a surface-based coordinate system,Neuro-image 9,195-207.
Hursh, J.B. (1939). Conduction velocity and diameter of nerve fibers. Am. J. Physiol. 127, 131–139.
Ishibashi, T., Dakin, K.A., Stevens, B., Lee, P.R., Kozlov, S.V., Stewart, C.L., and Fields, R.D. (2006). Astrocytes promote myelination in response to electrical impulses. Neuron 49, 823–832.
Itoh, K., Stevens, B., Schachner, M., and Fields, R.D. (1995). Regulated expression of the neural cell adhesion molecule L1 by specific patterns of neural impulses. Sci-ence 270, 1369–1372. Fischl, B., Sereno, M.I., Tootell, R.B., and Dale, A.M. (1999b). High-resolution intersubject averaging and a coordinate system for the cortical surface.
Hum. Brain Mapp. 8, 272–284. Itoh, K.,Ozaki,M., Stevens,B.,and Fields,R.D. (1997). Activity-dependent regulation of N-cadherin in DRG neurons:differential regulation of N-cadherin, NCAM, and L1 by distinct patterns of action potentials. J. Neurobiol. 33, 735 – 748.
Fischl, B., Stevens, A.A., Rajendran, N., Yeo, B.T., Greve, D.N., Van Leemput, K., Polimeni, J.R., Kakunoori, S., Buckner, R.L., Pacheco, J., et al. (2009). Predicting the location of entorhinal cortex from MRI. Neuroimage 47, 8–17. Jadi, M.P., and Sejnowski, T.J. (2014). Cortical oscillations arise from contextual interactions that regulate sparse coding. Proc. Natl. Acad. Sci.USA 111, 6780–6785.
Frühbeis,C., Fröhlich,D., Kuo,W.P., Amphornrat,J., Thilemann, S., Saab, A.S., Kirch- hoff,F., Möbius,W., Goebbels,S., Nave,K.A., et al. (2013). Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 11, e1001604. Jourdain,P., Bergersen, L.H., Bhaukaurally, K., Bezzi, P., Santello, M., Domercq, M., Matute, C., Tonello, F., Gundersen, V., and Volterra, A. (2007).
Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 10, 331 – 339.
Funfschilling, U., Supplie, L.M., Mahad, D., Boretius, S., Saab, A.S., Edgar, J., Brinkmann, B.G., Kassmann, C.M., Tzvetanova, I.D., Mobius, W., et al. (2012).
Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521. Kanemaru, K., Okubo, Y., Hirose,K., and Iino,M. (2007). Regulation of neurite growth by spontaneous Ca2+ oscillations in astrocytes. J. Neurosci. 27, 8957 – 8966.
Gärtner, H., Minnerop, M., Pieperhoff, P., Schleicher, A., Zilles,K., Altenmüller,E., and Amunts, K. (2013).Brain morphometry shows effects of long-term musical practice in middle-aged keyboard players. Front. Psychol. 4, 636.
Giaume, C.,Fromaget,C.,el Aoumari,A., Cordier,J., Glowinski,J., and Gros,D. (1991). Gap junctions in cultured astrocytes: single-channel currents and characterization of channel-forming protein. Neuron 6, 133–143.
Gibson, E.M., Purger, D., Mount, C.W.,Goldstein, A.K.,Lin, G.L., Wood, L.S., Inema, I., Miller, S.E., Bieri, G., Zuchero, J.B., et al. (2014). Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304.
Gómez-Casati, M. E., Murtie, J.C., Rio, C., Stankovic,K.,Liberman, M.C. and Corfas, G. (2010). Nonneuronal cells regulate synapse formation in the vestibular sensory epithelium via erbB-dependent BDNF expression. Proc. Natl. Acad. Sci. USA 107, 17005 – 17010.
Gordon, G.R., Choi,H.B., Rungta,R.L., Ellis-Davies,G.C., and MacVicar, B.A. (2008). Brain metabolism dictates the polarity of astrocyte control over arterioles.Nature 456, 745 – 749.
Halassa, M.M., Florian, C., Fellin, T., Munoz, J.R., Lee, S.Y., Abel, T., Haydon, P.G., and Frank, M.G. (2009). Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61, 213–219.
Han, J., Kesner, P., Metna-Laurent, M., Duan, T., Xu, L., Georges,F., Koehl, M., Ab- rous, D.N., Mendizabal-Zubiaga, J., Grandes, P., et al. (2012). Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 148, 1039–1050.
Han, X., Chen, M., Wang, F., Windrem, M., Wang, S., Shanz, S., Xu, Q., Oberheim, N.A., Bekar,L., Betstadt, S., et al. (2013). Forebrain engraftment by human glial pro- genitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12, 342–353.
Haustein, M.D., Kracun, S., Lu, X.-H., Shih, T., Jackson-Weaver,O., Tong,X., Xu, J., Yang, X.W., O’Dell, T.J., Marvin, J.S., et al. (2014). Conditions and constraints for astrocyte calcium signaling in the hippocampal mossy fiber pathway. Neuron 82, 413 – 429.
Henneberger, C., Papouin, T., Oliet, S.H., and Rusakov, D.A. (2010). Long-term potentiation depends on release of D-serine from astrocytes. Nature 463, 232 – 236. Neuron 86, April 22, 2015 a2015 Elsevier Inc.
384
Kettenman, H., and Ransom, B.R. (2013). Neuroglia, Third Edition. (Oxford, UK: Oxford University Press).
Kleinberger, G., Yamanishi, Y., Sua ́rez-Calvet, M., Czirr, E., Lohmann, E., Cuyvers, E., Struyfs, H., Pettkus, N., Wenninger-Weinzierl, A., Mazaheri, F., et al. (2014). TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci. Transl. Med. 6, 243ra86.
Kleinfeld, D., Bharioke, A., Blinder, P., Bock, D.D., Briggman, K.L., Chklovskii, D.B., Denk, W., Helmstaedter, M., Kaufhold, J.P., Lee, W.C., et al. (2011). Large-scale automated histology in the pursuit of connectomes. J. Neurosci. 31, 16125–16138.
Lalo, U., Palygin, O., Rasooli-Nejad, S., Andrew, J., Haydon, P.G., and Pankratov, Y. (2014). Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol. 12, e1001747.
Leblond, C.S., Kaneb, H.M., Dion, P.A., and Rouleau, G.A. (2014). Dissection of genetic factors associated with amyotrophic lateral sclerosis. Exp. Neurol. 262 Pt. B, 91 – 101.
Lee, S., Yoon, B.E., Berglund, K., Oh, S.J., Park,H., Shin,H.S., Augustine, G.J., and Lee, C.J. (2010). Channel-mediated tonic GABA release from glia. Science 330, 790 – 796.
Lee, H. S., Ghetti, A., Pinto-Duarte, A., Wang, X., Dziewczapolski, G., Galimi, F., Huitron-Resendiz, S., Pina-Crespo, J.C., Roberts, A.J., Verma, I.M., et al. (2014). Astrocytesic contribute to gamma oscillations and recognition memory. Proc. Natl. Acad. Sci. USA 111, E3343–E3352.
Lichtman, J.W., and Denk, W. (2011). The big and the small: challenges of imaging the brain’s circuits. Science 334, 618–623.
Lind, B.L., Brazhe, A.R., Jessen, S.B., Tan, F.C., and Lauritzen, M.J. (2013). Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc. Natl. Acad. Sci. USA 110, E4678–E4687.
Liu, J., Dietz, K., DeLoyht, J.M., Pedre, X., Kelkar, D., Kaur, J., Vialou, V., Lobo, M. K., Dietz,D.M., Nestler,E.J., et al. (2012). Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 15, 1621–1623.
Lundgaard, I., Luzhynskaya, A., Stockley, J.H., Wang, Z., Evans, K.A., Swire, M., Volbracht, K., Gautier, H.O., Franklin, R.J., Attwell,D., and Ka ́rado ́ttir, R.T.; Charles Ffrench-Constant (2013). Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 11, e1001743. Palmer, L.M., and Stuart,G.J. (2006). Site of action potential initiation in layer 5 pyramidal neurons. J. Neurosci. 26, 1854–1863.
385
Maglione, M., Tress, O., Haas, B., Karram, K., Trotter, J., Willecke, K., and Ketten-mann, H. (2010). Oligodendrocytes in mouse corpus callosum are coupled via gap junction channels formed by connexin47 and connexin32. Glia 58, 1104–1117.
Panatier,A., Theodosis,D.T., Mothet,J.P., Touquet, B., Pollegioni, L., Poulain, D.A., and Oliet, S.H. (2006). Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 125, 775–784.
Makinodan, M., Rosen, K.M., Ito, S., and Corfas,G. (2012). A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337, 1357 – 1360.
Mauch, D.H., Nägler, K., Schumacher, S., Go ̈ritz, C., Müller, E.C., Otto, A., and Pfrieger, F.W. (2001). CNS synaptogenesis promoted by glia-derived cholesterol. Science 294, 1354–1357.
Panatier, A., Vallée, J., Haber,M., Murai,K.K., Lacaille,J.C., and Robitaille, R. (2011). Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146, 785–798.
Pannasch, U., Vargová ,L., Reingruber,J.,Ezan,P., Holcman,D., Giaume,C., Syková, E., and Rouach, N. (2011). Astroglial networks scale synaptic activity and plasticity. Proc. Natl. Acad.Sci. USA 108, 8467–8472. Mizuseki, K., and Buzsáki, G. (2014). Theta oscillations decrease spike synchrony in the hippocampus and entorhinal cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20120530.
Paolicelli, R.C., Bolasco, G., Pagani, F., Maggi, L., Scianni, M., Panzanelli, P., Giustetto, M., Ferreira, T.A., Guiducci, E., Dumas, L., et al. (2011). Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458.
Molofsky, A.V., Krencik, R., Ullian, E.M., Tsai, H.H., Deneen, B., Richardson, W.D., Barres, B.A., and Rowitch, D.H. (2012). Astrocytes and disease: a neuro-develop-mental perspective. Genes Dev. 26, 891–907. Parpura, V., Basarsky, T.A., Liu, F., Jeftinija, K., Jeftinija, S., and Haydon, P.G. (1994). Glutamate-mediated astrocyte-neuron signalling. Nature 369, 744–747.
Molofsky, A.V., Kelley, K.W., Tsai, H.H., Redmond, S.A., Chang, S.M., Madireddy, L., Chan, J.R., Baranzini, S.E., Ullian, E.M., and Rowitch, D.H. (2014). Astrocyte-enco-ded positional cues maintain sensorimotor circuit integrity. Nature 509, 189–194.
Pascual, O., Casper, K.B., Kubera, C., Zhang, J., Revilla-Sanchez, R., Sul, J.Y., Takano, H., Moss, S.J., McCarthy, K., and Haydon, P.G. (2005). Astrocytic purinergic signaling coordinates synaptic networks. Science 310, 113–116.
Moore, C.I., and Cao, R. (2008). The hemo-neural hypothesis: on the role of blood flow in information processing. J. Neurophysiol. 99, 2035–2047.
Mu, Y., and Poo, M.M. (2006). Spike timing-dependent LTP/LTD mediates visual experience-dependent plasticity in a developing retinotectal system. Neuron 50, 115 – 125.
Mulligan, S.J., and MacVicar, B.A. (2004). Calcium transients in astrocyte end-feet cause cerebrovascular constrictions. Nature 431, 195–199.
Murai, K.K., Nguyen, L.N., Irie, F., Yamaguchi,Y., and Pasquale,E.B. (2003). Control of hippocampal dendritic spine morphology through ephrin-A3/ EphA4 signaling. Nat. Neurosci. 6, 153–160.
Navarrete, M., and Araque, A. (2010). Endocannabinoids potentiate synaptic trans-mission through stimulation of astrocytes. Neuron 68, 113–126.
Navarrete, M., Perea, G., Fernandez de Sevilla, D., Go ́mez-Gonzalo, M., Nunez, A., Martı ́n, E.D., and Araque, A. (2012). Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol. 10, e1001259.
Nedergaard, M. (1994). Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science 263, 1768–1771.
Nicholson, C., and Syková, E. (1998). Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 21, 207–215.
Nishida, H., and Okabe, S. (2007). Direct astrocytic contacts regulate local maturation of dendritic spines. J. Neurosci. 27, 331–340.
Nualart-Marti, A., Solsona, C., and Fields, R.D. (2013). Gap junction communication in myelinating glia. Biochim. Biophys. Acta 1828, 69–78.
Nunez, P.L., Srinivasan,R., and Fields,R.D. (2015).EEG functional connectivity, axon delays and white matter disease. Clin. Neurophysiol. 126, 110–120.
Oberheim, N.A., Wang, X., Goldman, S., and Nedergaard, M. (2006). Astrocytic complexity distinguishes the human brain. Trends Neurosci. 29, 547–553.
Owen, J.P., Li, Y.-O., Yang, F.G., Shetty, C., Bukshpun, P., Vora, S., Wakahiro, M., Hinkley, L.B., Nagarajan, S.S., Sherr, E.H., and Mukherjee, P. (2013). Resting-state networks and the functional connectome of the human brain in agenesis of the corpus callosum. Brain Connect. 3, 547–562.
Özarslan, E., Koay, C.G., Shepherd, T.M., Komlosh,M.E., Irfanog lu, M.O, Pierpaoli, C., and Basser, P.J. (2013). Mean apparent propagator (MAP) MRI: a novel diffusion imaging method for mapping tissue microstructure. Neuroimage 78, 16–32.
Pajevic, S., Basser, P.J., and Fields, R.D. (2014). Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience 276, 135–147.
Perea, G., and Araque, A. (2007). Astrocytes potentiate transmitter release at single hippocampal synapses. Science 317, 1083–1086.
Poskanzer, K.E., and Yuste, R. (2011). Astrocytic regulation of cortical UP states. Proc. Natl. Acad. Sci. USA 108, 18453–18458.
Pusic, A.D., and Kraig, R.P. (2014). Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia 62, 284 – 299.
Rouach, N., Koulakoff, A., Abudara, V., Willecke, K., and Giaume, C. (2008). Astro-glial metabolic networks sustain hippocampal synaptic transmission. Science 322, 1551 – 1555.
Sasaki, T., Matsuki, N., and Ikegaya, Y. (2011). Action-potential modulation during axonal conduction. Science 331, 599–601.
Sasaki, T., Beppu, K., Tanaka, K.F., Fukazawa, Y., Shigemoto, R., and Matsui, K. (2012). Application of an optogenetic byway for perturbing neuronal activity via glial photostimulation. Proc. Natl. Acad. Sci. USA 109, 20720–20725.
Schafer, D.P., Lehrman,E.K., Kautzman,A.G., Koyama,R., Mardinly,A.R., Yamasaki, R., Ransohoff, R.M.,Greenberg,M.E., Barres,B.A., and Stevens,B. (2012). Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705.
Schmandke, A., Schmandke, A., and Schwab,M.E. (2014). Nogo-A: Multiple roles in CNS development, maintenance, and disease. Neuroscientist 20, 372–386.
Serrano, A., Haddjeri,N., Lacaille,J.C., and Robitaille, R. (2006). GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression.J. Neurosci. 26, 5370–5382.
Shigetomi, E., Jackson-Weaver, O., Huckstepp, R.T., O’Dell, T.J., and Khakh, B.S. (2013). TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J. Neurosci. 33, 10143–10153.
Smit, D.J., Boersma, M., Schnack, H.G., Micheloyannis, S., Boomsma, D.I., Hulshoff Pol, H.E., Stam, C.J., and de Geus,E.J.(2012).The brain matures with stronger func- tional connectivity and decreased randomness of its network. PLoS ONE 7, e36896.
Somogyi, P., Tamas, G., Lujan,R., and Buhl,E.H. (1998). Salient features of synaptic organisation in the cerebral cortex. Brain Res. Brain Res. Rev. 26, 113–135.
Stanford, L.R. (1987). Conduction velocity variations minimize conduction time differences among retinal ganglion cell axons. Science 238, 358–360.
Stellwagen, D., and Malenka, R.C. (2006). Synaptic scaling mediated by glial TNF-alpha. Nature 440, 1054–1059.
386
Stevens, B., and Fields, R.D. (2000). Response of Schwann cells to action potentials in development. Science 287, 2267–2271.
Stevens, B., Tanner, S., and Fields, R.D. (1998). Control of myelination by specific patterns of neural impulses. J. Neurosci. 18, 9303–9311.
Stevens, B., Porta, S., Haak, L.L., Gallo, V., and Fields, R.D. (2002). Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron 36, 855–868.
Stikov, N., Perry, L.M., Mezer, A., Pauly, J.M., Wandell, B.A., and Dougherty, R.F. (2011). Modeling and measuring the myelin g-ratio. Proc. Intl. Soc. Magn. Reson. Med. 19, 225.
Stork, T., Sheehan, A., Tasdemir-Yilmaz, O.E., and Freeman, M.R. (2014). Neuron-glia interactions through the Heartless FGF receptor signaling pathway mediate morphogenesis of Drosophila astrocytes. Neuron 83, 388–403.
Sul, J.-Y., Orosz, G., Givens, R.S., and Haydon, P.G. (2004). Astrocytic connectivity in the hippocampus. Neuron Glia Biol. 1, 3–11.
Suzuki, A., Stern, S.A., Bozdagi, O., Huntley, G.W., Walker, R.H., Magistretti, P.J., and Alberini, C.M. (2011). Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144, 810–823.
Swadlow, H.A., Rosene, D.L., and Waxman, S.G. (1978). Characteristics of inter-hemispheric impulse conduction between prelunate gyri of the rhesus monkey. Exp. Brain Res. 33, 455–467.
Tasaki, I., Ishii, K., and Ito, H. (1943). On the relation between the conduction- rate, the fiber-diameter and the internodal distance of the medullated nerve fiber. Jpn. J. Med. Sci. Biophys. 9, 189–199.
Tasdemir-Yilmaz, O.E., and Freeman, M.R. (2014). Astrocytes engage unique mole-cular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev. 28, 20–33.
Tomassy, G.S., Berger, D.R., Chen, H.H., Kasthuri, N., Hayworth, K.J., Vercelli, A., Seung, H.S., Lichtman, J.W., and Arlotta, P. (2014). Distinct profiles of myelin distri-bution along single axons of pyramidal neurons in the neocortex. Science 344, 319 – 324.
Tremblay, M.E., Lowery, R.L., and Majewska, A.K.(2010). Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 8, e1000527.
Wake, H., Moorhouse, A.J., Jinno, S., Kohsaka, S., and Nabekura,J. (2009). Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 29, 3974–3980.
Wake, H., Lee, P.R., and Fields, R.D. (2011). Control of local protein synthesis and initial events in myelination by action potentials. Science 333,1647–1651. Neuron 86, April 22,2015 a2015 Elsevier Inc.
386
Wang, X., Lou, N., Xu, Q., Tian, G.F., Peng, W.G., Han,X., Kang,J., Takano, T., and Nedergaard, M. (2006). Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat.Neurosci. 9,816–823.
Wang, F., Smith, N.A., Xu,Q., Fujita,T., Baba,A.,Matsuda,T., Takano,T., Bekar,L., and Nedergaard, M. (2012). Astrocytes modulate neural network activity by Ca 2+ -dependent uptake of extracellular K+. Sci. Signal. 5, ra26.
Waxman, S.G.(1980).Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve 3, 141–150.
Waxman, S.G., and Bennett, M.V. (1972). Relative conduction velocities of small myelinated and non-myelinated fibres in the central nervous system. Nat. New Biol. 238, 217–219.
Wedeen, V.J., Wang, R.P., Schmahmann,J.D., Benner, T., Tseng, W.Y., Dai, G., Pan- dya, D.N., Hagmann, P., D’Arceuil, H., and de Crespigny, A.J.(2008). Diffusion spect- rum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage 41, 1267–1277.
Woo, D.H., Han, K.S., Shim, J.W., Yoon, B.E., Kim, E., Bae, J.Y., Oh, S.J., Hwang, E.M., Marmorstein,A.D., Bae,Y.C.,et al. (2012).TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation.Cell 151, 25-40.
Xie, A.X., Lauderdale, K., Murphy, T., Myers, T.L., and Fiacco, T.A. (2014). Inducing plasticity of astrocytic receptors my manipulation of neuronal firing rates. J. Vis. Exp. http://dx.doi.org/10.3791/51458.
Yakovlev, P.I., and Lecours, A.R. (1967). The myelogenetic cycles of regional matu-ration of the brain. In Regional Development of the Brain in Early Life, A. Minkowski, ed. (Oxford, UK: Blackwell), pp. 3–70.
Yamazaki, Y., Hozumi, Y., Kaneko, K., Sugihara, T., Fujii, S., Goto, K., and Kato, H. (2007). Modulatory effects of oligodendrocytes on the conduction velocity of action potentials along axons in the alveus of the rat hippocampal CA1 region. Neuron Glia Biol. 3, 325–334.
Young,K.M., Psachoulia,K.,Tripathi, R.B., Dunn,S.J., Cossell,L., Attwell,D., Tohyama, K.,and Richardson, W.D.(2013).Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885.
Yuen,T.J.,Silbereis,J.C., Griveau,A., Chang, S.M., Daneman, R., Fancy, S.P., Zahed, H., Maltepe, E., and Rowitch, D.H. (2014). Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis.Cell 158, 383 – 396.
Zatorre, R.J., Fields, R.D., and Johansen-Berg,H.(2012). Plasticity in gray and white: neuroimaging changes in brain structure during learning.Nat. Neurosci. 15, 528–536.
Zhang, J., Malik, A., Choi, H.B., Ko, R.W.Y., Dissing-Olesen, L., and MacVicar, B.A. (2014). Microglial CR3 activation triggers long-term synaptic depression in the hippocampus via NADPH oxidase. Neuron 82, 195–207.
Kommentit